Abstract
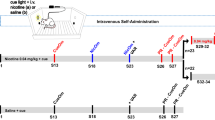
Although established smokers have a very regular pattern of smoking behavior, converging lines of evidence suggest that the escalation of smoking behavior is a critical factor in the development of dependence. However, the neurobiological mechanisms that underlie the escalation of smoking are unknown, because there is no animal model of the escalation of nicotine intake. On the basis of the pattern of smoking behavior in humans and presence of monoamine oxidase inhibitors in tobacco smoke, we hypothesized that the escalation of nicotine intake may only occur when animals are given extended-access (21 h per day) self-administration sessions after repeated periods of abstinence (24–48 h), and after chronic inhibition of monoamine oxidase using phenelzine sulfate. Intermittent access (every 24–48 h) to extended nicotine self-administration produced a robust escalation of nicotine intake, associated with increased responding under fixed- and progressive-ratio schedules of reinforcement, and increased somatic signs of withdrawal. The escalation of nicotine intake was not observed in rats with intermittent access to limited (1 h per day) nicotine self-administration or daily access to extended (21 h per day) nicotine self-administration. Moreover, inhibition of monoamine oxidase with daily administration of phenelzine increased nicotine intake by ∼50%. These results demonstrate that the escalation of nicotine intake only occurs in animals given intermittent periods of abstinence with extended access to nicotine, and that inhibition of monoamine oxidase may contribute to the escalation of smoking, thus validating both an animal model of the escalation of smoking behavior and the contribution of monoamine oxidase inhibition to compulsive nicotine-seeking.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300.
Ahmed SH, Walker JR, Koob GF (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421.
Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A (2004). The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res 995: 46–54.
Berlin I, Anthenelli RM (2001). Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol 4: 33–42.
Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, Marcus MD (2005). The return to smoking: 1-year relapse trajectories among female smokers. Nicotine Tob Res 7: 533–540.
Cummings KM, Mahoney M (2006). Current and emerging treatment approaches for tobacco dependence. Curr Oncol Rep 8: 475–483.
Doubeni CA, Reed G, Difranza JR (2010). Early course of nicotine dependence in adolescent smokers. Pediatrics 125: 1127–1133.
Dougherty J, Miller D, Todd G, Kostenbauder HB (1981). Reinforcing and other behavioral effects of nicotine. Neurosci Biobehav Rev 5: 487–495.
Engelmann JM, Radke AK, Gewirtz JC (2009). Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology (Berl) 207: 13–25.
Epping-Jordan MP, Watkins SS, Koob GF, Markou A (1998). Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393: 76–79.
Fowler JS, Logan J, Wang GJ, Volkow ND, Telang F, Zhu W et al (2003). Low monoamine oxidase B in peripheral organs in smokers. Proc Natl Acad Sci USA 100: 11600–11605.
Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R et al (1996). Inhibition of monoamine oxidase B in the brains of smokers. Nature 379: 733–736.
George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH et al (2007). CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA 104: 17198–17203.
Greenwell TN, Funk CK, Cottone P, Richardson HN, Chen SA, Rice KC et al (2009). Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long-, but not short-access rats. Addict Biol 14: 130–143.
Grunberg NE (1994). Overview: biological processes relevant to drugs of dependence. Addiction 89: 1443–1446.
Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M et al (2005). Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci 25: 8593–8600.
Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M et al (2006). Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci 24: 3532–3540.
Heilig M, Koob GF (2007). A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30: 399–406.
Henningfield JE, Goldberg SR (1983). Nicotine as a reinforcer in human subjects and laboratory animals. Pharmacol Biochem Behav 19: 989–992.
Hughes JR, Higgins ST, Bickel WK (1994). Nicotine withdrawal vs other drug withdrawal syndromes: similarities and dissimilarities. Addiction 89: 1461–1470.
Irvine EE, Cheeta S, File SE (2001). Tolerance to nicotine's effects in the elevated plus-maze and increased anxiety during withdrawal. Pharmacol Biochem Behav 68: 319–325.
Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL (2000). Nicotine blood levels and sublective craving for cigarettes. Pharmacol Biochem Behav 66: 553–558.
Kapelewski CH, Vandenbergh DJ, Klein LC (2011). Effect of the monoamine oxidase inhibition on rewarding effects of nicotine in rodents. Curr Drug Abuse Rev 4: 110–121.
Kenny PJ, Markou A (2006). Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31: 1203–1211.
Kim MJ, Fleming CB, Catalano RF (2009). Individual and social influences on progression to daily smoking during adolescence. Pediatrics 124: 895–902.
Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L (2006). Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology (Berl) 186: 48–53.
Koob GF (2010). The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314: 3–14.
Koob GF, Le Moal M (2001). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24: 97–129.
Kozlowski LT, Wilkinson DA, Skinner W, Kent C, Franklin T, Pope M (1989). Comparing tobacco cigarette dependence with other drug dependencies: greater or equal ‘difficulty quitting’ and ‘urges to use,’ but less ‘pleasure’ from cigarettes. JAMA 261: 898–901.
LeSage MG, Burroughs D, Pentel PR (2006). Effects of nicotine withdrawal on performance under a progressive-ratio schedule of sucrose pellet delivery in rats. Pharmacol Biochem Behav 83: 585–591.
Lotfipour S, Arnold MM, Hogenkamp DJ, Gee KW, Belluzzi JD, Leslie FM (2011). The monoamine oxidase (MAO) inhibitor tranylcypromine enhances nicotine self-administration in rats through a mechanism independent of MAO inhibition. Neuropharmacology 61: 95–104.
Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA et al (1992). Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav 43: 779–784.
McManus DJ, Greenshaw AJ (1991). Differential effects of antidepressants on GABAB and β-adrenergic receptors in rat cerebral cortex. Biochem Pharmacol 42: 1525–1528.
O’Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE et al (2007). Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther 320: 180–193.
O’Dell LE, Koob GF (2007). ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav 86: 346–353.
Paterson NE, Markou A (2004). Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 173: 64–72.
Peto R, Lopez AD, Boreham J, Thun M, Heath Jr C, Doll R (1996). Mortality from smoking worldwide. Br Med Bull 52: 12–21.
Pomerleau CS, Pomerleau OF (1992). Euphoriant effects of nicotine in smokers. Psychopharmacology (Berl) 108: 460–465.
Richardson NR, Roberts DCS (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11.
Risner ME, Goldberg SR (1983). A comparison of nicotine and cocaine self-administration in the dog: fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther 224: 319–326.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R et al (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32: 1816–1823.
Solomon RL, Corbit JD (1973). An opponent-process theory of motivation: II. Cigarette addiction. J Abnorm Psychol 81: 158–171.
Stolerman IP, Jarvis MJ (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117: 2–10; discussion, 14–20.
Torrijos RM, Glantz SA (2006). The US Public Health Service ‘treating tobacco use and dependence clinical practice guidelines’ as a legal standard of care. Tob Control 15: 447–451.
US Department of Health and Human Services (1988). The Health Consequences of Smoking: Nicotine Addiction. A Report of the Surgeon General, 1988 (DHHS Publication no. [CDC] 88-8406). Centers for Disease Control and Prevention, Office on Smoking and Health: Rockville.
Valentine JD, Hokanson JS, Matta SG, Sharp BM (1997). Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 133: 300–304.
Villégier AS, Blanc G, Glowinski J, Tassin JP (2003). Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav 76: 267–274.
Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM et al (2006). Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology 31: 1704–1713.
Acknowledgements
This is publication number 21726 from The Scripps Research Institute. This work was supported by the Tobacco-Related Disease Research Program (TRDRP) from the State of California (Grant 17RT-0095), Pearson Center for Alcoholism and Addiction Research, and National Institute on Drug Abuse (DA023597). This work also was based on discussions and support from the Tobacco Etiology Research Network (TERN) of the Robert Wood Johnson foundation. We thank Michael Arends for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Cohen, A., Koob, G. & George, O. Robust Escalation of Nicotine Intake with Extended Access to Nicotine Self-Administration and Intermittent Periods of Abstinence. Neuropsychopharmacol 37, 2153–2160 (2012). https://doi.org/10.1038/npp.2012.67
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.67
Keywords
This article is cited by
-
Intermittent nicotine access is as effective as continuous access in promoting nicotine seeking and taking in rats
Psychopharmacology (2024)
-
Altered neuronal activity in the ventromedial prefrontal cortex drives nicotine intake escalation
Neuropsychopharmacology (2023)
-
Flavor additives facilitate oral self-administration of nicotine solution in mice
Psychopharmacology (2021)
-
Non-genetic factors that influence methamphetamine intake in a genetic model of differential methamphetamine consumption
Psychopharmacology (2020)
-
Nicotine e-cigarette vapor inhalation effects on nicotine & cotinine plasma levels and somatic withdrawal signs in adult male Wistar rats
Psychopharmacology (2020)