Abstract
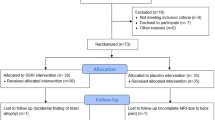
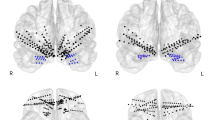
The amygdala is a key structure in the pathophysiology of anxiety disorders, and a putative target for anxiolytic treatments. Selective serotonin reuptake inhibitors (SSRIs) and placebo seem to induce anxiolytic effects by attenuating amygdala responsiveness. However, conflicting amygdala findings have also been reported. Moreover, the neural profile of responders and nonresponders is insufficiently characterized and it remains unknown whether SSRIs and placebo engage common or distinct amygdala subregions or different modulatory cortical areas. We examined similarities and differences in the neural response to SSRIs and placebo in patients with social anxiety disorder (SAD). Positron emission tomography (PET) with oxygen-15-labeled water was used to assess regional cerebral blood flow (rCBF) in 72 patients with SAD during an anxiogenic public speaking task, before and after 6–8 weeks of treatment under double-blind conditions. Response rate was determined by the Clinical Global Impression-Improvement scale. Conjunction analysis revealed a common rCBF-attenuation from pre- to post-treatment in responders to SSRIs and placebo in the left basomedial/basolateral and right ventrolateral amygdala. This rCBF pattern correlated with behavioral measures of reduced anxiety and differentiated responders from nonresponders. However, nonanxiolytic treatment effects were also observed in the amygdala. All subgroups, including nonresponders, showed deactivation of the left lateral part of the amygdala. No rCBF differences were found between SSRI responders and placebo responders. This study provides new insights into the brain dynamics underlying anxiety relief by demonstrating common amygdala targets for pharmacologically and psychologically induced anxiety reduction, and by showing that the amygdala is functionally heterogeneous in anxiolysis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aggleton JP (1985). A description of intra-amygdaloid connections in old world monkeys. Exp Brain Res 57: 390–399.
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders (DSM IV) 4th edn American Psychiatric Press: Washington, DC.
Bach DR, Behrens TE, Garrido L, Weiskopf N, Dolan RJ (2011). Deep and superficial amygdala nuclei projections revealed in vivo by probabilistic tractography. J Neurosci 31: 618–623.
Bar M (2009). A cognitive neuroscience hypothesis of mood and depression. Trends Cogn Sci 13: 456–463.
Benedetti F, Arduino C, Costa S, Vighetti S, Tarenzi L, Rainero I et al (2006). Loss of expectation-related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain 121: 133–144.
Benedetti F, Colloca L, Torre E, Lanotte M, Melcarne A, Pesare M et al (2004). Placebo-responsive Parkinson patients show decreased activity in single neurons of subthalamic nucleus. Nat Neurosci 7: 587–588.
Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK (2005). Neurobiological mechanisms of the placebo effect. J Neurosci 25: 10390–10402.
Bigos KL, Pollock BG, Aizenstein HJ, Fisher PM, Bies RR, Hariri AR (2008). Acute 5-HT reuptake blockade potentiates human amygdala reactivity. Neuropsychopharmacology 33: 3221–3225.
Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I (2005). Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry 57: 832–840.
Bueno CH, Zangrossi H, Viana MB (2005). The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz J Med Biol Res 38: 1697–1701.
Cheng L-L, Wang S-J, Gean P-W (1998). Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci 10: 2163–2172.
Colloca L, Lopiano L, Lanotte M, Benedetti F (2004). Overt vs covert treatment for pain, anxiety and Parkinson's disease. Lancet Neurol 3: 679–684.
Colloca L, Miller FG (2011). Role of expectations in health. Curr Opin Psychiatry 24: 149–155.
Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH (2010). Neural correlates of rumination in depression. Cogn Affect Behav Neurosci 10: 470–478.
Davidson JRT (2006). Pharmacotherapy of social anxiety disorder: what does the evidence tell us? J Clin Psychiatry 67: 20–26.
Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ (2010). Regional response differences across the human amygdaloid complex during social conditioning. Cereb Cortex 20: 612–621.
Domschke K, Dannlowski U (2009). Imaging genetics of anxiety disorders. NeuroImage 15: 822–831.
Eippert F, Finsterbusch J, Bingel U, Büchel C (2009). Direct evidence for spinal cord involvement in placebo analgesia. Science 16: 404.
Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 6: 1361–1372.
Evans KC, Dougherty DD, Pollack MH, Rauch SL (2006). Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry 18: 33–42.
Faria V, Fredrikson M, Furmark T (2008). Imaging the placebo response: a neurofunctional review. Eur Neuropsychopharmacol 18: 473–485.
First M, Gibbon M, Spitzer R, Williams J (1998). SCID-I: Interview Protocol (in Swedish). Pilgrim Press: Stockholm.
Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC et al (2010). Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303: 47–53.
François C, Despiégel N, Maman K, Saragoussi D, Auquier P (2010). Anxiety disorders, major depressive disorder and the dynamic relationship between these conditions: treatment patterns and cost analysis. J Med Econ 13: 99–109.
Fredman SJ, Fava M, Kienke AS, White CN, Nierenberg AA, Rosenbaum JF (2000). Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: A survey of current ‘next-step’ practices. J Clin Psychiatry 61: 403–408.
Fuente-Fernández R de la, Schulzer M, Stoessl AJ (2004). Placebo mechanisms and reward circuitry: clues from Parkinson's disease. Biol Psychiatry 56: 67–71.
Furmark T, Appel L, Henningsson S, Åhs F, Faria V, Linnman C et al (2008). A link between serotonin-related gene polymorphisms, amygdala activity, and placebo-induced relief from social anxiety. J Neurosci 28: 13066–13074.
Furmark T, Appel L, Michelgård A, Wahlstedt K, Åhs F, Zancan S et al (2005). Cerebral blood flow changes after treatment of social phobia with the neurokinin-1 antagonist GR205171, citalopram, or placebo. Biol Psychiatry 58: 132–142.
Furmark T, Henningsson S, Appel L, Åhs F, Linnman C, Pissiota A et al (2009). Genotype over-diagnosis in amygdala responsiveness: affective processing in social anxiety disorder. J Psychiatry Neurosci 34: 30–40.
Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A, Långström B et al (2002). Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 59: 425–433.
Goldin PR, McRae K, Ramel W, Gross JJ (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 63: 577–586.
Green S, Vale AL (1992). Role of amygdaloid nuclei in the anxiolytic effects of benzodiazepines in rats. Behav Pharmacol 3: 261–264.
Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM (2006). Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry 59: 816–820.
Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S et al (2004). Selective serotonin reuptake inhibitor reduces conditioned fear through its effect in the amygdala. Eur J Pharmacol 497: 311–316.
Izumi T, Inoue T, Kitaichi Y, Nakagawa S, Koyama T (2006). Target brain sites of the anxiolytic effect of citalopram, a selective serotonin reuptake inhibitor. Eur J Psychopharmacol 534: 129–132.
Kessler RC (2003). The impairments caused by social phobia in the general population: implications for intervention. Acta Psychiatr Scand 108: 19–27.
Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 617–627.
Khan A, Bhat A (2008). Is the problem of a high placebo response unique to antidepressant trials? J Clin Psychiatry 69: 1979–1980.
Khan A, Leventhal RM, Khan SR, Brown WA (2002). Severity of depression and response to antidepressants and placebo: an analysis of the Food and Drug Administration database. J Clin Psychopharmacol 22: 40–45.
Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE et al (2006). The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology 31: 2243–2253.
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT (2008). Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 5: e45.
Kirsch I, Sapirstein G (1998). Listening to Prozac but hearing placebo: a meta-analysis of antidepressant medication. Prev Treat 6: 1–16.
Krummenacher P, Candia V, Folkers G, Schedlowski M, Schönbächler G (2010). Prefrontal cortex modulates placebo analgesia. Pain 48: 368–374.
Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al (2000). Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131.
Lázaro-Muñoz G, LeDoux JE, Cain CK (2010). Sidman instrumental avoidance initially depends on lateral and basal amygdala and is constrained by central amygdala-mediated Pavlovian processes. Biol Psychiatry 67: 1120–1127.
LeDoux J (2007). The Amygdala. Curr Biol 17: R868–R874.
Leuchter AF, Cook IA, Witte EA, Morgan M, Abrams M (2002). Changes in brain function of depressed subjects during treatment with placebo. Am J Psychiatry 159: 122–129.
Lidstone SC, Schulzer M, Dinelle K, Mak E, Sossi V, Ruth TJ et al (2010). Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch Gen Psychiatry 67: 857–865.
Liebowitz MR (1987). Social phobia. Mod Probl Pharmacopsychiatry 22: 141–173.
Mai JK, Assheuer J, Paxinos G (2004). Atlas of the Human Brain. Elsevier Academic: San Diego.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19: 1233–1239.
Maslowsky J, Mogg K, Bradley BP, McClure-Tone E, Ernst M, Pine DS et al (2010). A preliminary investigation of neural correlates of treatment in adolescents with generalized anxiety disorder. J Child Adol Psychopharmacol 20: 105–111.
Mathew SJ, Price RB, Charney DS (2008). Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. Am J Med Genetics 148: 89–98.
Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S et al (2002). The functional neuroanatomy of the placebo effect. Am J Psychiatry 159: 728–737.
Moncrieff J, Wessely S, Hardy R (2004). Active placebos vs antidepressants for depression. Cochrane Database Syst Rev 1: CD003012.
Morris JS, Buchel C, Dolan RJ (2001). Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. NeuroImage 13: 1044–1052.
Murphy SE, Norbury R, Sullivan UO, Cowen PJ, Harmer CJ (2009). Effect of a single dose of citalopram on amygdala response to emotional faces. Br J Psychiatry 194: 535–540.
Niehoff DL, Kuhar MJ (1983). Benzodiazepine receptors: localization in rat amygdala. J Neurosci 3: 2091–2097.
Ochsner KN, Gross JJ (2005). The cognitive control of emotion. Trends Cogn Sci 9: 242–249.
Pazos A, Probst A, Palacios JM (1987). Serotonin receptors in the human brain-III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21: 97–122.
Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M (2005). Placebo in emotional processing-induced expectations of anxiety relief activate a generalized modulatory network. Neuron 46: 957–969.
Petrovic P, Kalso E, Petersson KM, Ingvar M (2002). Placebo and opioid analgesia. Imaging a shared neuronal network. Science 295: 1737–1740.
Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I (2006). Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry 63: 184–192.
Phelps EA, LeDoux JE (2005). Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48: 175–187.
Pitkänen A, Savander V, LeDoux JE (1997). Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 20: 517–523.
Ravindran LN, Stein MB (2010). The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry 71: 839–854.
Ressler KJ (2010). Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry 67: 1117–1119.
Rosen HJ, Levenson RW (2009). The emotional brain: combining insights from patients and basic science. Neurocase 15: 173–181.
Rourke HO, Fudge JL (2006). Distribution of serotonin transporter labeled fibers in distribution of serotonin transporter labeled fibers in amygdaloid subregions: implications for mood disorders. Biol Psychiatry 60: 479–490.
Roy AK, Shehzad Z, Marqulies DS, Kelly AM, Uddin LQ, Gotimer K et al (2010). Functional connectivity of the human amygdala using resting state fMRI. NeuroImage 45: 614–626.
Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK (2007). Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55: 325–336.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22–33.
Shin LM, Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacol 35: 169–191.
Spielberger CD, Gorsuch RL, Lushene RE (1970). Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA.
Straube T, Glauer M, Dilger S, Mentzel H-J, Miltner WHR (2006). Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage 29: 125–135.
Stutzmann GE, McEwen BS, LeDoux JE (1998). Serotonin modulation of sensory inputs to the lateral amygdala: dependency on corticosterone. J Neurosci 18: 9529–9538.
Talairach J, Tournoux P (1988). Co-Planar Stereotaxic Atlas of the Human Brain. Georg Thiene Verlag: Stuttgart, Germany.
Telles-Correia D, Guerreiro DF, Oliveira S, Figueira ML (2007). Differences between SSRI's pharmacokinetics and pharmacodynamics. Acta Med Port 20: 167–174.
Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H et al (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362.
Uhlenhuth EH, Matuzas W, Warner TD, Thompson PM (1997). Growing placebo response rate: the problem in recent therapeutic trials? Psychopharmacol Bull 33: 31–39.
Van Ameringen M, Mancini C, Farvolden P, Oakman J (2000). Drugs in development for social anxiety disorder: more to social anxiety than meets the SSRI. Expert Opin Investig Drugs 9: 2215–2231.
Yonkers KA, Dyck IR, Keller MB (2001). An eight-year longitudinal comparison of clinical course and characteristics of social phobia among men and women. Psychiatric Services 52: 637–643.
Zaider TI, Heimberg RG, Fresco DM, Scheiner FR, Liebowitz MR (2003). Evaluation of the clinical global impression scale among individuals with social anxiety disorder. Psychol Med 33: 611–622.
Zubieta JK, Stohler CS (2009). Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci 1156: 198–210.
Acknowledgements
This work was supported by GlaxoSmithKline, the Swedish Research Council, the Swedish Council for Working Life and Social Research, and a grant from the Foundation of Science and Technology from the Portuguese Ministry of Science, Technology and Higher Education co-financed by the European Social funding. We thank all patients for their contribution to this study and the staff members of Uppsala PET centre and Quintiles for providing excellent research conditions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Massimo Bani, Paolo Bettica, and Emilio Merlo Pich were full-time employees at GlaxoSmithKline at the time of the design and conduct of this study. None of the other authors declare conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Faria, V., Appel, L., Åhs, F. et al. Amygdala Subregions Tied to SSRI and Placebo Response in Patients with Social Anxiety Disorder. Neuropsychopharmacol 37, 2222–2232 (2012). https://doi.org/10.1038/npp.2012.72
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2012.72
Keywords
This article is cited by
-
Altered hippocampus and amygdala subregion connectome hierarchy in major depressive disorder
Translational Psychiatry (2022)
-
Nucleus accumbens volume as a predictor of anxiety symptom improvement following CBT and SSRI treatment in two independent samples
Neuropsychopharmacology (2020)
-
Developmental Pathways from Early Behavioral Inhibition to Later Anxiety: An Integrative Review of Developmental Psychopathology Research and Translational Implications
Adolescent Research Review (2019)
-
Neuroimaging Predictors and Mechanisms of Treatment Response in Social Anxiety Disorder: an Overview of the Amygdala
Current Psychiatry Reports (2018)
-
Mechanisms of the placebo effect in pain and psychiatric disorders
The Pharmacogenomics Journal (2016)