Abstract
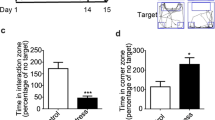
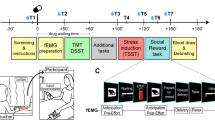
Stress is implicated in diverse psychiatric disorders including substance abuse. The locus coeruleus–norepinephrine (LC–NE) system is a major stress response system that is also a point of intersection between stress neuromediators and endogenous opioids and so may be a site at which stress can influence drug-taking behaviors. As social stress is a common stressor for humans, this study characterized the enduring impact of repeated social stress on LC neuronal activity. Rats were exposed to five daily consecutive sessions of social stress using the resident-intruder model or control manipulation. LC discharge rate recorded 2 days after the last manipulation was decreased in stressed rats compared with controls. By 10 days after the last manipulation, LC rates were comparable between groups. Systemic administration of the opiate antagonist, naloxone, robustly increased LC discharge rate in a manner suggestive of opiate withdrawal, selectively in stressed rats when administered 2 or 10 days after the last manipulation. This was accompanied by behavioral signs of mild opiate withdrawal. Western blot and electron microscopic studies indicated that repeated social stress decreased corticotropin-releasing factor type 1 receptor and increased μ-opioid receptor levels in the LC. Together, the results suggest that repeated social stress engages endogenous opioid modulation of LC activity and induces signs of cellular and physical opiate dependence that endure after the stress. These cellular effects may predispose individuals with a history of repeated social stress to substance abuse behaviors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aghajanian GK (1978). Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature 276: 186–188.
Akaoka H, Aston-Jones G (1991). Opiate withdrawal-induced hyperactivity of locus coeruleus neurons is substantially mediated by augmented excitatory amino acid input. J Neurosci 11: 3830–3839.
Aston-Jones G, Hirata H, Akaoka H (1997). Local opiate withdrawal in locus coeruleus in vivo. Brain research 765: 331–336.
Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H et al (2010). Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15: 896–904.
Berenz EC, Coffey SF (2012). Treatment of co-occurring posttraumatic stress disorder and substance use disorders. Curr Psychiatry Rep 14: 469–477.
Caille S, Espejo EF, Reneric JP, Cador M, Koob GF, Stinus L (1999). Total neurochemical lesion of noradrenergic neurons of the locus ceruleus does not alter either naloxone-precipitated or spontaneous opiate withdrawal nor does it influence ability of clonidine to reverse opiate withdrawal. J Pharmacol Exp Ther 290: 881–892.
Chan RKW, Sawchenko PE (1994). Spatially and temporally differentiated patterns of c-fos expression in brainstem catecholaminergic cell groups induced by cardiovascular challenges in the rat. J Comp Neurol 348: 433–460.
Conti LH, Foote SL (1995). Effects of pretreatment with corticotropin-releasing factor on the electrophysiological responsivity of the locus coeruleus to subsequent corticotropin-releasing factor challenge. Neuroscience 69: 209–219.
Conti LH, Foote SL (1996). Reciprocal cross-desensitization of locus coeruleus electrophysiological responsivity to corticotropin-releasing factor and stress. Brain Res 722: 19–29.
Cruz FC, Quadros IM, Hogenelst K, Planeta CS, Miczek KA (2011). Social defeat stress in rats: escalation of cocaine and ‘speedball’ binge self-administration, but not heroin. Psychopharmacology 215: 165–175.
Curtis AL, Bello NT, Valentino RJ (2001). Endogenous opioids in the locus coeruleus function to limit the noradrenergic response to stress. J Neurosci 21: RC152.
Curtis AL, Florin-Lechner SM, Pavcovich LA, Valentino RJ (1997). Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther 281: 163–172.
Curtis AL, Leiser SC, Snyder K, Valentino RJ (2012). Predator stress engages corticotropin-releasing factor and opioid systems to alter the operating mode of locus coeruleus norepinephrine neurons. Neuropharmacology 62: 1737–1745.
Delfs JM, Zhu Y, Druhan JP, Aston-Jones G (2000). Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403: 430–434.
Faravelli C, Lo Sauro C, Lelli L, Pietrini F, Lazzeretti L, Godini L et al (2012). The role of life events and HPA axis in anxiety disorders: a review. Curr Pharmaceutical Design 18: 5663–5674.
Funk CK, Zorrilla EP, Lee MJ, Rice KC, Koob GF (2007). Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry 61: 78–86.
Gold PW, Chrousos GP (2002). Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psych 7: 254–275.
Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ et al (2006). Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behaviors. J Neurosci 26: 4624–4629.
Heim C, Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry 49: 1023–1039.
Jedema HP, Grace AA (2004). Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci 24: 9703–9713.
Kreibich A, Reyes BA, Curtis AL, Ecke L, Chavkin C, Van Bockstaele EJ et al (2008). Presynaptic inhibition of diverse afferents to the locus ceruleus by kappa-opiate receptors: a novel mechanism for regulating the central norepinephrine system. J Neurosci 28: 6516–6525.
Maldonado R, Stinus L, Gold LH, Koob GF (1992). Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J Pharmacol Exp Ther 261: 669–677.
Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ (1994). mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain research 643: 245–265.
McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci 840: 33–44.
Miczek KA (1979). A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 60: 253–259.
Miczek KA (1991). Tolerance to the analgesic, but not discriminative stimulus effects of morphine after brief social defeat in rats. Psychopharmacology 104: 181–186.
Miczek KA, Covington HE 3rd, Nikulina EM Jr, Hammer RP (2004). Aggression and defeat: persistent effects on cocaine self-administration and gene expression in peptidergic and aminergic mesocorticolimbic circuits. Neurosci Biobehav Rev 27: 787–802.
Miczek KA, Thompson ML, Shuster L (1986). Analgesia following defeat in an aggressive encounter: development of tolerance and changes in opioid receptors. Ann N Y Acad Sci 467: 14–29.
Mirzaii-Dizgah I, Karimian SM, Hajimashhadi Z, Riahi E, Ghasemi T (2008). Attenuation of morphine withdrawal signs by muscimol in the locus coeruleus of rats. Behav Pharmacol 19: 171–175.
Punch LJ, Self DW, Nestler EJ, Taylor JR (1997). Opposite modulation of opiate withdrawal behaviors on microinfusion of a protein kinase A inhibitor versus activator into the locus coeruleus or periaqueductal gray. J Neurosci 17: 8520–8527.
Rasmussen K, Aghajanian GK (1989). Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res 505: 346–350.
Rasmussen K, Beitner-Johnson DB, Krystal JG, Aghajanian GK, Nestler EJ (1990). Opiate withdrawal and rat locus coeruleus: behavioral, electophysiological and biochemical correlates. J Neurosci 10: 2308–2317.
Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ (2006). Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci 23: 2991–2998.
Reyes BA, Valentino RJ, Van Bockstaele EJ (2008). Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology 149: 122–130.
Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Minarro J (2006). Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology 185: 459–470.
Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U (2005). Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162: 127–134.
Schiff M, Levit S, Cohen-Moreno R (2010). Childhood sexual abuse, post-traumatic stress disorder, and use of heroin among female clients in Israeli methadone maintenance treatment programs (MMTPS). Social Work Health Care 49: 799–813.
Smagin GN, Swiergiel AH, Dunn AJ (1994). Sodium nitroprusside infusions activate cortical and hypothalamic noradrenergic systems in rats. Neurosci Res Comm 14: 85–91.
Southwick SM, Bremner JD, Rasmusson A, Morgan CA 3rd, Arnsten A, Charney DS (1999). Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry 46: 1192–1204.
Taylor JR, Punch LJ, Elsworth JD (1998). A comparison of the effects of clonidine and CNQX infusion into the locus coeruleus and the amygdala on naloxone-precipitated opiate withdrawal in the rat. Psychopharmacology (Berl) 138: 133–142.
Taylor PJ, Gooding P, Wood AM, Tarrier N (2011). The role of defeat and entrapment in depression, anxiety, and suicide. Psychological Bull 137: 391–420.
Valentino RJ, Page ME, Curtis AL (1991). Activation of noradrenergic locus coeruleus neurons by hemodynamic stress is due to local release of corticotropin-releasing factor. Brain Res 555: 25–34.
Valentino RJ, Van Bockstaele E (2008). Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583: 194–203.
Valentino RJ, Wehby RG (1989). Locus coeruleus discharge characteristics of morphine-dependent rats: Effects of naltrexone. Brain Res 488: 126–134.
Van Bockstaele EJ, Branchereau P, Pickel VM (1995). Morphologically heterogeneous met-enkephalin terminals form synapses with tyrosine hydroxylase containing dendrites in the rat nucleus locus coeruleus: an immuno-electron microscopic analysis. J Comp Neurol 363: 423–438.
Van Bockstaele EJ, Colago EE, Valentino RJ (1996). Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol 364: 523–534.
Williams JT, North RA (1984). Opiate receptor interactions on single locus coeruleus neurons. Mol Pharm 26: 489–497.
Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P et al (2000). Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA 97: 325–330.
Wood SK, Walker HE, Valentino RJ, Bhatnagar S (2010). Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology 151: 1795–1805.
Xu GP, Van Bockstaele E, Reyes B, Bethea T, Valentino RJ (2004). Chronic morphine sensitizes the brain norepinephrine system to corticotropin-releasing factor and stress. J Neurosci 24: 8193–8197.
Acknowledgements
The authors acknowledge the technical assistance of Elizabeth Verhoeve and Sandra Luz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Chaijale, N., Curtis, A., Wood, S. et al. Social Stress Engages Opioid Regulation of Locus Coeruleus Norepinephrine Neurons and Induces a State of Cellular and Physical Opiate Dependence. Neuropsychopharmacol 38, 1833–1843 (2013). https://doi.org/10.1038/npp.2013.117
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.117
Keywords
This article is cited by
-
Neurochemically distinct circuitry regulates locus coeruleus activity during female social stress depending on coping style
Brain Structure and Function (2019)
-
Region-specific roles of the corticotropin-releasing factor–urocortin system in stress
Nature Reviews Neuroscience (2016)
-
Adolescent Social Stress Produces an Enduring Activation of the Rat Locus Coeruleus and Alters its Coherence with the Prefrontal Cortex
Neuropsychopharmacology (2016)
-
Stress and Drug Dependence Differentially Modulate Norepinephrine Signaling in Animals with Varied HPA Axis Function
Neuropsychopharmacology (2015)
-
Repeated Social Stress Increases Reward Salience and Impairs Encoding of Prediction by Rat Locus Coeruleus Neurons
Neuropsychopharmacology (2015)