Abstract
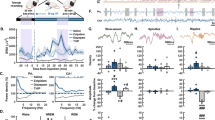
Dual orexin receptor antagonists (DORAs) induce sleep by blocking orexin 1 and orexin 2 receptor-mediated activities responsible for regulating wakefulness. DORAs represent a potential alternative mechanism to the current standard of care that includes the γ-aminobutyric acid (GABA)A receptor-positive allosteric modulators, eszopiclone and zolpidem. This work uses an innovative method to analyze electroencephalogram (EEG) spectral frequencies within sleep/wake states to differentiate the effects of GABAA modulators from DORA-22, an analog of the DORA MK-6096, in Sprague–Dawley rats. The effects of low, intermediate, and high doses of eszopiclone, zolpidem, and DORA-22 were examined after first defining each compound’s ability to promote sleep during active-phase dosing. The EEG spectral frequency power within specific sleep stages was calculated in 1-Hz intervals from 1 to 100 Hz within each sleep/wake state for the first 4 h after the dose. Eszopiclone and zolpidem produced marked, dose-responsive disruptions in sleep stage-specific EEG spectral profiles compared with vehicle treatment. In marked contrast, DORA-22 exhibited marginal changes in the spectral profile, observed only during rapid eye movement sleep, and only at the highest dose tested. Moreover, while eszopiclone- and zolpidem-induced changes were evident in the inactive period, the EEG spectral responses to DORA-22 were absent during this phase. These results suggest that DORA-22 differs from eszopiclone and zolpidem whereby DORA-22 promotes somnolence without altering the neuronal network EEG activity observed during normal sleep.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Achermann P, Borbely AA (1987). Dynamics of EEG slow wave activity during physiological sleep and after administration of benzodiazepine hypnotics. Hum Neurobiol 6: 203–210.
Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbely AA (1994). Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacology 11: 237–244.
Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ (2012a). Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology 37: 1224–1233.
Bettica P, Squassante L, Groeger JA, Gennery B, Winsky-Sommerer R, Dijk DJ (2012b). Differential effects of a dual orexin receptor antagonist (SB-649868) and zolpidem on sleep initiation and consolidation, SWS, REM sleep, and EEG power spectra in a model of situational insomnia. Neuropsychopharmacology 37: 1224–1233.
Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S et al (2007). Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med 13: 150–155.
Brunner DP, Dijk DJ, Munch M, Borbely AA (1991). Effect of zolpidem on sleep and sleep EEG spectra in healthy young men. Psychopharmacology (Berl) 104: 1–5.
Coleman PJ, Schreier JD, Cox CD, Breslin MJ, Whitman DB, Bogusky MJ et al (2012). Discovery of [(2R,5R)-5-{[(5-fluoropyridin-2-yl)oxy]methyl}-2-methylpiperidin-1-yl][5-methyl-2-(pyrimidin-2-yl)phenyl]methanone (MK-6096): a dual orexin receptor antagonist with potent sleep-promoting properties. Chem Med Chem 7: 415–424 337.
Cox CD, Breslin MJ, Whitman DB, Schreier JD, McGaughey GB, Bogusky MJ et al (2010). Discovery of the dual orexin receptor antagonist [(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone (MK-4305) for the treatment of insomnia. J Med Chem 53: 5320–5332.
Desarnaud F, Murillo-Rodriguez E, Lin L, Xu M, Gerashchenko D, Shiromani SN et al (2004). The diurnal rhythm of hypocretin in young and old F344 rats. Sleep 27: 851–856.
Espa F, Ondze B, Deglise P, Billiard M, Besset A (2000). Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol 111: 929–939.
Feige B, Voderholzer U, Riemann D, Hohagen F, Berger M (1999). Independent sleep EEG slow-wave and spindle band dynamics associated with 4 weeks of continuous application of short-half-life hypnotics in healthy subjects. Clin Neurophysiol 110: 1965–1974.
Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ (2012). International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev 64: 389–420.
Herring WJ, Snyder E, Budd K, Hutzelmann J, Snavely D, Liu K et al (2012). Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology 79: 2265–2274.
Hindmarch I, Legangneux E, Stanley N, Emegbo S, Dawson J (2006). A double-blind, placebo-controlled investigation of the residual psychomotor and cognitive effects of zolpidem-MR in healthy elderly volunteers. Br J Clin Pharmacol 62: 538–545.
Hoque R, Chesson AL Jr (2009). Zolpidem-induced sleepwalking, sleep related eating disorder, and sleep-driving: fluorine-18-flourodeoxyglucose positron emission tomography analysis, and a literature review of other unexpected clinical effects of zolpidem. J Clin Sleep Med 5: 471–476.
Karni A, Tanne D, Rubenstein BS, Askenasy JJ, Sagi D (1994). Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265: 679–682.
Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J et al (2002). Release of hypocretin (orexin) during waking and sleep states. J Neurosci 22: 5282–5286.
Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR (2002). NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 25: 630–640.
Landolt HP, Finelli LA, Roth C, Buck A, Achermann P, Borbely AA (2000). Zolpidem and sleep deprivation: different effect on EEG power spectra. J Sleep Res 9: 175–183.
Lundahl J, Deacon S, Maurice D, Staner L (2012). EEG spectral power density profiles during NREM sleep for gaboxadol and zolpidem in patients with primary insomnia. J Psychopharmacol 26: 1081–1087.
Martinez GS, Smale L, Nunez AA (2002). Diurnal and nocturnal rodents show rhythms in orexinergic neurons. Brain Res 955: 1–7.
Mintzer MZ, Frey JM, Yingling JE, Griffiths RR (1997). Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol 8: 561–574.
Ohayon MM (2002). Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 6: 97–111.
Otmani S, Demazieres A, Staner C, Jacob N, Nir T, Zisapel N et al (2008). Effects of prolonged-release melatonin, zolpidem, and their combination on psychomotor functions, memory recall, and driving skills in healthy middle aged and elderly volunteers. Hum Psychopharmacol 23: 693–705.
Renger JJ, Dunn SL, Motzel SL, Johnson C, Koblan KS (2004). Sub-chronic administration of zolpidem affects modifications to rat sleep architecture. Brain Res 1010: 45–54.
Rush CR, Armstrong DL, Ali JA, Pazzaglia PJ (1998). Benzodiazepine-receptor ligands in humans: acute performance-impairing, subject-rated and observer-rated effects. J Clin Psychopharmacol 18: 154–165.
Saper CB, Scammell TE, Lu J (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437: 1257–1263.
Smith CT, Nixon MR, Nader RS (2004). Posttraining increases in REM sleep intensity implicate REM sleep in memory processing and provide a biological marker of learning potential. Learn Mem 11: 714–719.
Spiegelhalder K, Regen W, Feige B, Holz J, Piosczyk H, Baglioni C et al (2012). Increased EEG sigma and beta power during NREM sleep in primary insomnia. Biol Psychol 91: 329–333.
Trachsel L, Dijk DJ, Brunner DP, Klene C, Borbely AA (1990). Effect of zopiclone and midazolam on sleep and EEG spectra in a phase-advanced sleep schedule. Neuropsychopharmacology 3: 11–18.
Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ et al (2011). Promotion of sleep by suvorexant—a novel dual orexin receptor antagonist. J Neurogenet 25: 52–61.
Winrow CJ, Gotter AL, Cox CD, Tannenbaum PL, Garson SL, Doran SM et al (2012). Pharmacological characterization of MK-6096—a dual orexin receptor antagonist for insomnia. Neuropharmacology 62: 978–987.
Acknowledgements
We acknowledge Drs Ellen Snyder, Vladimir Svetnik, Junshui Ma, and Shubhankar Ray for their analytic and statistical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Fox, S., Gotter, A., Tye, S. et al. Quantitative Electroencephalography Within Sleep/Wake States Differentiates GABAA Modulators Eszopiclone and Zolpidem From Dual Orexin Receptor Antagonists in Rats. Neuropsychopharmacol 38, 2401–2408 (2013). https://doi.org/10.1038/npp.2013.139
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.139
Keywords
This article is cited by
-
Nonclinical pharmacology of daridorexant: a new dual orexin receptor antagonist for the treatment of insomnia
Psychopharmacology (2021)
-
Neurophysiological and Behavioral Effects of Anti-Orexinergic Treatments in a Mouse Model of Huntington's Disease
Neurotherapeutics (2019)
-
Orexin Receptor Antagonists
Current Sleep Medicine Reports (2017)
-
Orexin 2 Receptor Antagonism is Sufficient to Promote NREM and REM Sleep from Mouse to Man
Scientific Reports (2016)
-
Differential sleep-promoting effects of dual orexin receptor antagonists and GABAAreceptor modulators
BMC Neuroscience (2014)