Abstract
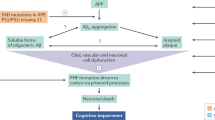
Drug candidates directed against amyloid-β (Aβ) are mainstream in Alzheimer’s disease (AD) drug development. Active and passive Aβ immunotherapy is the principle that has come furthest, both in number and in stage of clinical trials. However, an increasing number of reports on major difficulties in identifying any clinical benefit in phase II–III clinical trials on this type of anti-Aβ drug candidates have caused concern among researchers, pharmaceutical companies, and other stakeholders. This has provided critics of the amyloid cascade hypothesis with fire for their arguments that Aβ deposition may merely be a bystander, and not the cause, of the disease or that the amyloid hypothesis may only be valid for the familial form of AD. On the other hand, most researchers argue that it is the trial design that will need refinement to allow for identifying a positive clinical effect of anti-Aβ drugs. A consensus in the field is that future trials need to be performed in an earlier stage of the disease and that biomarkers are essential to guide and facilitate drug development. In this context, it is reassuring that, in contrast to most brain disorders, research advances in the AD field have led to both imaging (magnetic resonance imaging (MRI) and PET) and cerebrospinal fluid (CSF) biomarkers for the central pathogenic processes of the disease. AD biomarkers will have a central role in future clinical trials to enable early diagnosis, and Aβ biomarkers (CSF Aβ42 and amyloid PET) may be essential to allow for testing a drug on patients with evidence of brain Aβ pathology. Pharmacodynamic Aβ and amyloid precursor protein biomarkers will be of use to verify target engagement of a drug candidate in humans, thereby bridging the gap between mechanistic data from transgenic AD models (that may not be relevant to the neuropathology of human AD) and large and expensive phase III trials. Last, downstream biomarker evidence (CSF tau proteins and MRI volumetry) that the drug ameliorates neurodegeneration will, together with beneficial clinical effects on cognition and functioning, be essential for labeling an anti-Aβ drug as disease modifying.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC et al (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 270–279.
Andersson M, Alvarez-Cermeño J, Bernardi G, Cogato I, Fredman P, Frederiksen J et al (1994). Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 57: 897–902.
Andreasen N, Minthon L, Vanmechelen E, Vanderstichele H, Davidsson P, Winblad B et al (1999). CSF-tau and CSF-Aβ42 as predictors of development of Alzheimer’s disease in patients with mild cognitive impairment. Neurosci Lett 273: 5–8.
Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B et al (2001). Evaluation of CSF-tau and CSF-Aβ42 as diagnostic markers for Alzheimer’s disease in clinical practice. Arch Neurol 58: 373–379.
Andreasen N, Blennow K, Zetterberg H (2010). Neuroinflammation screening in immunotherapy trials against Alzheimer’s disease. Int J Alzheimers Dis 2010: 638379.
Alzheimer’s Prevention Initiative (2013). Alzheimer’s Prevention Initiative—Treatment Trials. http://endalznow.org/about-api/treatment-trials.aspx.
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H et al (2000). Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916–919.
Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L et al (2005). Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology 64: 94–101.
Bazenet C, Lovestone S (2012). Plasma biomarkers for Alzheimer’s disease: much needed but tough to find. Biomark Med 6: 441–454.
Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsäter H, Anckarsäter R et al (2010). Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 986310.
Black RS, Sperling RA, Safirstein B, Motter RN, Pallay A, Nichols A et al (2011). A single ascending dose study of bapineuzumab in patients with Alzheimer disease. Alzheimer Dis Assoc Disord 24: 198–203.
Blennow K, Fredman P, Wallin A, Gottfries CG, Karlsson I, Långström L et al (1993). Protein analyses in cerebrospinal fluid: II. Reference values derived from healthy individuals 18–88 years of age. Eur Neurol 33: 129–133.
Blennow K (2005). CSF biomarkers for Alzheimer’s disease: use in early diagnosis and evaluation of drug treatment. Expert Rev Mol Diagn 5: 661–672.
Blennow K, De Leon MJ, Zetterberg H (2006). Alzheimer’s disease. Lancet 368: 387–403.
Blennow K, Zetterberg H, Minthon L, Lannfelt L, Strid S, Annas P et al (2007). Longitudinal stability of CSF biomarkers in Alzheimer’s disease. Neurosci Lett 419: 18–22.
Blennow K (2010). Biomarkers in Alzheimer’s disease drug development. Nat Med 16: 1218–1222.
Blennow K, Hampel H, Weiner M, Zetterberg H (2010). Cerebrospinal fluid and plasma biomarkers in Alzheimer’s disease. Nat Rev Neurol 6: 131–144.
Blennow K, Zetterberg H, Rinne JO, Salloway S, Wei J, Black R et al AAB-001 201/202 Investigators (2012). Effect of immunotherapy with bapineuzumab on cerebrospinal fluid biomarker levels in patients with mild to moderate Alzheimer disease. Arch Neurol 69: 1002–1010.
Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F et al (2012). Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J Alzheimers Dis 28: 49–69.
Burstein AH, Zhao Q, Ross J, Styren S, Landen JW, Ma WW et al (2013). Safety and pharmacology of ponezumab (PF-04360365) after a single 10-minute intravenous infusion in subjects with mild to moderate Alzheimer disease. Clin Neuropharmacol 36: 8–13.
ClinicalTrials.gov (2013). A service of the U.S. National Institutes of Health. Alzheimer Disease Trials online http://www.clinicaltrials.gov/ct2/results?cond=%22Alzheimer+Disease%22.
Davidsson P, Blennow K, Andreasen N, Eriksson B, Minthon L, Hesse C (2001). Differential increase in cerebrospinal fluid-acetylcholine esterase after treatment with acetylcholine esterase inhibitors in patients with Alzheimer’s disease. Neurosci Lett 300: 157–160.
DeCarli C (2003). Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol 2: 15–21.
Degerman Gunnarsson M, Lindau M, Wall A, Blennow K, Darreh-Shori T, Basu S et al (2010). Pittsburgh compound-B and Alzheimer’s disease biomarkers in CSF, plasma and urine—an exploratory study. Dement Geriatr Cogn Disord 29: 204–212.
Delrieu J, Ousset PJ, Vellas B (2012). Gantenerumab for the treatment of Alzheimer’s disease. Expert Opin Biol Ther 12: 1077–1086.
DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM (2001). Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA 98: 8850–8855.
Dodel R, Hampel H, Depboylu C, Lin S, Gao F, Schock S et al (2002). Human antibodies against amyloid beta peptide: a potential treatment for Alzheimer’s disease. Ann Neurol 52: 253–256.
Dodel R, Rominger A, Bartenstein P, Barkhof F, Blennow K, Förster S et al (2013). Intravenous immunoglobulin for treatment of mild-to-moderate Alzheimer’s disease: a phase 2, randomised, double-blind, placebo-cntrolled, dose-finding trial. Lancet Neurol 12: 233–243.
Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J et al (2007). Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS–ADRDA criteria. Lancet Neurol 6: 734–746.
Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR et al (2006). Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 59: 512–519.
Fagan T (2012). Bapineuzumab phase 3: target engagement, but no benefit. Alzheimer Research Forum online http://www.alzforum.org/new/detail.asp?id=3268.
Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP et al (2012). Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement 8: 261–271.
FDA—US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) (2013). Guidance for Industry. Alzheimer’s Disease: Developing Drugs for the Treatment of Early Stage Disease. Draft Guidance online http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM338287.pdf.
Fierce Biotech (2012). Researchers Announce Treatment Choice for Alzheimer’s Disease (A4) Prevention Clinical Trial (online) http://www.fiercebiotech.com/press-releases/researchers-announce-treatment-choice-alzheimers-disease-a4-prevention-clin.
Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L et al AN1792(QS-21)-201 Study (2005). Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology 64: 1563–1572.
Frisoni GB, Blennow K (2013). Biomarkers for Alzheimer’s: the sequel of an original model. Lancet Neurol 12: 126–128.
Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC et al AN1792(QS-21)-201 Study Team (2005). Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 64: 1553–1562.
Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L et al (1991). Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349: 704–706.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E et al Alzheimer Genetic Analysis Group (2013). TREM2 variants in Alzheimer’s disease. N Engl J Med 368: 117–127.
Götz J, Ittner A, Ittner LM (2012). Tau-targeted treatment strategies in Alzheimer’s disease. Br J Pharmacol 165: 1246–1259.
Hampel H, Frank R, Broich K, Teipel SJ, Katz RG, Hardy J et al (2010). Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov 9: 560–574.
Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L (2006). Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 5: 228–234.
Hardy J, Selkoe DJ (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297: 353–356.
Hardy J (2009). The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem 110: 1129–1134.
Henriksen K, O’Bryant S, Hampel H, Trojanowski JQ, Montine TJ, Jeromin A et al (2013). The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement (in press).
Herholz K (2012). Use of FDG PET as an imaging biomarker in clinical trials of Alzheimer’s disease. Biomark Med 6: 431–439.
Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A et al (2008). Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet 372: 216–223.
Isaac M, Vamvakas S, Abadie E, Jonsson B, Gispen C, Pani L (2011). Qualification opinion of novel methodologies in the predementia stage of Alzheimer’s disease: cerebro-spinal-fluid related biomarkers for drugs affecting amyloid burden—regulatory considerations by European Medicines Agency focusing in improving benefit/risk in regulatory trials. Eur Neuropsychopharmacol 21: 781–788.
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS et al (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12: 207–216.
Jellinger KA (2008). Neuropathological aspects of Alzheimer disease, Parkinson disease and frontotemporal dementia. Neurodegener Dis 5: 118–121.
Johansson P, Mattsson N, Hansson O, Wallin A, Johansson JO, Andreasson U et al (2011). Cerebrospinal fluid biomarkers for Alzheimer’s disease: diagnostic performance in a homogeneous mono-center population. J Alzheimers Dis 24: 537–546.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J et al (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368: 107–116.
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH et al (1987). The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736.
Kotzbauer PT, Trojanowsk JQ, Lee VM (2001). Lewy body pathology in Alzheimer’s disease. J Mol Neurosci 17: 225–232.
Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F (2012). Plasma amyloid-β as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol 69: 824–831.
Kuo YM, Emmerling MR, Lampert HC, Hempelman SR, Kokjohn TA, Woods AS et al (1999). High levels of circulating Abeta42 are sequestered by plasma proteins in Alzheimer’s disease. Biochem Biophys Res Commun 257: 787–791.
La Porte SL, Bollini SS, Lanz TA, Abdiche YN, Rusnak AS, Ho WH et al (2012). Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer’s disease. J Mol Biol 421: 525–536.
Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CB Jr et al (2013). Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol 36: 14–23.
Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J et al PBT2-201-EURO Study Group (2008). Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol 7: 779–786.
Lee JM, Blennow K, Andreasen N, Laterza O, Modur V, Olander J et al (2008). The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem 54: 1617–1623.
Lemere CA, Masliah E (2010). Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nat Rev Neurol 6: 108–119.
Liu Y, Studzinski C, Beckett T, Murphy MP, Klein RL, Hersh LB (2010). Circulating neprilysin clears brain amyloid. Mol Cell Neurosci 45: 101–107.
Liu Z, Zhu H, Fang GG, Walsh K, Mwamburi M, Wolozin B et al (2012). Characterization of insulin degrading enzyme and other amyloid-β degrading proteases in human serum: a role in Alzheimer’s disease? J Alzheimers Dis 29: 329–340.
Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ (2012). Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol 8: 465–469.
Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA et al (1988). The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med 112: 634–640.
Mann DM, Yates PO, Marcyniuk B (1984). Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down’s syndrome in middle age form an age related continuum of pathological changes. Neuropathol Appl Neurobiol 10: 185–207.
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA 82: 4245–4249.
Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M et al (2009). CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302: 385–393.
Mattsson N, Andreasson U, Persson S, Albert M, Arai H, Batish D et al (2011). The Alzheimer’s Association external quality control program for CSF biomarkers. Alzheimers Dement 7: 386–395.
Mattsson N, Zegers I, Andreasson U, Bjerke M, Blankenstein MA, Bowser R et al (2012a). Reference measurement procedures for Alzheimer’s disease cerebrospinal fluid biomarkers: definitions and approaches with focus on amyloid β42. Biomark Med 6: 409–417.
Mattsson N, Andreasson U, Carrillo MC, Persson S, Shaw LM, Zegers I et al (2012b). Proficiency testing programs for Alzheimer’s disease cerebrospinal fluid biomarkers. Biomark Med 6: 401–407.
May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN et al (2011). Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci 31: 16507–16516.
Mehta PD, Pirttilä T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM (2000). Plasma and cerebrospinal fluid levels of amyloid beta proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 57: 100–105.
National Institute of Aging (2013). Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia (DIAN TU) online http://www.nia.nih.gov/alzheimers/clinical-trials/dominantly-inherited-alzheimer-network-trial-opportunity-prevent-dementia.
Nelson PT, Kukull WA, Frosch MP (2010). Thinking outside the box: Alzheimer-type neuropathology that does not map directly onto current consensus recommendations. J Neuropathol Exp Neurol 69: 449–454.
Noelker C, Hampel H, Dodel R (2011). Blood-based protein biomarkers for diagnosis and classification of neurodegenerative diseases: current progress and clinical potential. Mol Diagn Ther 15: 83–102.
Olsson B, Hertze J, Ohlsson M, Nägga K, Höglund K, Basun H et al (2012). Cerebrospinal fluid levels of heart fatty acid binding protein are elevated prodromally in Alzheimer’s disease and vascular dementia. J Alzheimers Dis 34: 673–679.
O’Nuallain B, Hrncic R, Wall JS, Weiss DT, Solomon A (2006). Diagnostic and therapeutic potential of amyloid-reactive IgG antibodies contained in human sera. J Immunol 176: 7071–7078.
Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC et al (2003). Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 61: 46–54.
Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ et al (2012). Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol 69: 198–207.
Otto M, Wiltfang J, Tumani H, Zerr I, Lantsch M, Kornhuber J et al (1997). Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Neurosci Lett 225: 210–212.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A et al (2011). Interacting with γ-secretase for treating Alzheimer’s disease: from inhibition to modulation. Curr Med Chem 18: 5430–5447.
Panza F, Frisardi V, Solfrizzi V, Imbimbo BP, Logroscino G, Santamato A et al (2012). Immunotherapy for Alzheimer’s disease: from anti-β-amyloid to tau-based immunization strategies. Immunotherapy 4: 213–238.
Prinz F, Schlange T, Asadullah K (2011). Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov 10: 712.
Quigley H, Colloby SJ, O'Brien JT (2011). PET imaging of brain amyloid in dementia: a review. Int J Geriatr Psychiatry 26: 991–999.
Randall J, Mörtberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S et al (2013). Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation 84: 351–356.
Reiman EM, Jagust WJ (2012). Brain imaging in the study of Alzheimer’s disease. Neuroimage 61: 505–516.
Relkin NR, Szabo P, Adamiak B, Burgut Tuna, Monthea C, Lent RW et al (2009). 18-Month study of intravenous immunoglobulin for treatment of mild Alzheimer disease. Neurobiol Aging 30: 1728–1736.
Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE et al (2010). (11)C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer’s disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol 9: 363–372.
Roth M, Tomlinson BE, Blessed G (1966). Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature 209: 109–110.
Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M et al Bapineuzumab 201 Clinical Trial Investigators (2009). A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology 73: 2061–2070.
Sämgård K, Zetterberg H, Blennow K, Hansson O, Minthon L, Londos E (2010). Cerebrospinal fluid total tau as a marker of Alzheimer’s disease intensity. Int J Geriatr Psychiatry 25: 403–410.
Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T et al (1999). Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173–177.
Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC et al Alzheimer’s Disease Neuroimaging Initiative (2009). Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 65: 403–413.
Singh S, Kushwah AS, Singh R, Farswan M, Kaur R (2012). Current therapeutic strategy in Alzheimer’s disease. Eur Rev Med Pharmacol Sci 16: 1651–1664.
Sperling R, Salloway S, Brooks DJ, Tampieri D, Barakos J, Fox NC et al (2012). Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 11: 241–249.
Steinacker P, Mollenhauer B, Bibl M, Cepek L, Esselmann H, Brechlin P et al (2004). Heart fatty acid binding protein as a potential diagnostic marker for neurodegenerative diseases. Neurosci Lett 370: 36–39.
Streffer JR, Blennow K, Salloway S, Zetterberg H, Xu YZ, Lu Y et al for the Bapineuzumab Phase 3 Investigator Group (2013). Effect of bapineuzumab on CSF P-tau and T-tau in mild to moderate Alzheimer’s disease: results from two phase 3 trials in APOE ɛ4 carriers and non-carriers. Abstract submitted to the AAIC meeting, Boston.
Strobel G (2013). DIAN Trial Picks Gantenerumab, Solanezumab, Maybe BACE Inhibitor (online) http://www.alzforum.org/new/detail.asp?id=3289.
Strobel G (2011). Anti-Amyloid Treatment in Asymptomatic AD Trial (online) http://www.alzforum.org/new/detail.asp?id=3014.
Strozyk D, Blennow K, White LR, Launer LJ (2003). CSF Aβ42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60: 652–656.
Szabo P, Relkin N, Weksler ME (2008). Natural human antibodies to amyloid beta peptide. Autoimmun Rev 7: 415–420.
Tibbling G, Link H, Ohman S (1977). Principles of albumin and IgG analyses in neurological disorders. I. Establishment of reference values. Scand J Clin Lab Invest 37: 385–390.
Visser PJ, Verhey F, Knol DL, Scheltens P, Wahlund LO, Freund-Levi Y et al (2009). Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol 8: 619–627.
Walker JR, Pacoma R, Watson J, Ou W, Alves J, Mason DE et al (2013). Enhanced proteolytic clearance of plasma aβ by peripherally administered neprilysin does not result in reduced levels of brain aβ in mice. J Neurosci 33: 2457–2464.
Wallin ÅK, Hansson O, Blennow K, Londos E, Minthon L (2009). Can CSF biomarkers or pre-treatment progression rate predict response in cholinesterase inhibitor treatment in Alzheimer’s disease? Int J Geriatr Psychiatry 24: 638–647.
Weksler ME, Relkin N, Turkenich R, LaRusse S, Zhou L, Szabo P (2002). Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp Gerontol 37: 943–948.
Wiessner C, Wiederhold KH, Tissot AC, Frey P, Danner S, Jacobson LH et al (2011). The second-generation active Aβ immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J Neurosci 31: 9323–9331.
Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T et al (2012). Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol 11: 597–604.
Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S et al (2009). Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci 29: 11393–11398.
Zago W, Buttini M, Comery TA, Nishioka C, Gardai SJ, Seubert P et al (2012). Neutralization of soluble, synaptotoxic amyloid β species by antibodies is epitope specific. J Neurosci 32: 2696–2702.
Zahs KR, Ashe KH (2010). ‘Too much good news’—are Alzheimer mouse models trying to tell us how to prevent, not cure, Alzheimer’s disease? Trends Neurosci 33: 381–389.
Zetterberg H, Pedersen M, Lind K, Svensson M, Rolstad S, Eckerström C et al (2007). Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis 12: 255–260.
Zetterberg H, Tullhög K, Hansson O, Minthon L, Londos E, Blennow K (2010). Low incidence of post-lumbar puncture headache in 1089 consecutive memory clinic patients. Eur Neurol 63: 326–330.
Zetterberg H, Wilson D, Andreasson U, Minthon L, Blennow K, Randall J et al (2013). Plasma tau levels in Alzheimer’s disease. Alzheimers Res Ther 5: 9.
Acknowledgements
Professor Blennow receives research support by the Swedish Research Council (Grant No. 14002). Prof. Hampel receives research support by the Katharina-Hardt Foundation, Bad Homburg, Germany.
Author information
Authors and Affiliations
Corresponding author
PowerPoint slides
Rights and permissions
About this article
Cite this article
Blennow, K., Hampel, H. & Zetterberg, H. Biomarkers in Amyloid-β Immunotherapy Trials in Alzheimer’s Disease. Neuropsychopharmacol 39, 189–201 (2014). https://doi.org/10.1038/npp.2013.154
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.154
Keywords
This article is cited by
-
Nanomedicine-based immunotherapy for central nervous system disorders
Acta Pharmacologica Sinica (2020)
-
Urinary metabolic phenotyping for Alzheimer’s disease
Scientific Reports (2020)
-
The impact of transcranial magnetic stimulation on diagnostic confidence in patients with Alzheimer disease
Alzheimer's Research & Therapy (2018)
-
Co-Administration of TiO2 Nanowired Mesenchymal Stem Cells with Cerebrolysin Potentiates Neprilysin Level and Reduces Brain Pathology in Alzheimer’s Disease
Molecular Neurobiology (2018)
-
Factors that influence the levels of cerebrospinal fluid biomarkers in memory clinic patients
BMC Geriatrics (2017)