Abstract
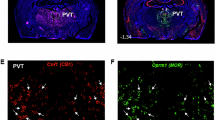
This study was aimed to evaluate the involvement of CB2 cannabinoid receptors (CB2r) in the rewarding, reinforcing and motivational effects of nicotine. Conditioned place preference (CPP) and intravenous self-administration experiments were carried out in knockout mice lacking CB2r (CB2KO) and wild-type (WT) littermates treated with the CB2r antagonist AM630 (1 and 3 mg/kg). Gene expression analyses of tyrosine hydroxylase (TH) and α3- and α4-nicotinic acetylcholine receptor subunits (nAChRs) in the ventral tegmental area (VTA) and immunohistochemical studies to elucidate whether CB2r colocalized with α3- and α4-nAChRs in the nucleus accumbens and VTA were performed. Mecamylamine-precipitated withdrawal syndrome after chronic nicotine exposure was evaluated in CB2KO mice and WT mice treated with AM630 (1 and 3 mg/kg). CB2KO mice did not show nicotine-induced place conditioning and self-administered significantly less nicotine. In addition, AM630 was able to block (3 mg/kg) nicotine-induced CPP and reduce (1 and 3 mg/kg) nicotine self-administration. Under baseline conditions, TH, α3-nAChR, and α4-nAChR mRNA levels in the VTA of CB2KO mice were significantly lower compared with WT mice. Confocal microscopy images revealed that CB2r colocalized with α3- and α4-nAChRs. Somatic signs of nicotine withdrawal (rearings, groomings, scratches, teeth chattering, and body tremors) increased significantly in WT but were absent in CB2KO mice. Interestingly, the administration of AM630 blocked the nicotine withdrawal syndrome and failed to alter basal behavior in saline-treated WT mice. These results suggest that CB2r play a relevant role in the rewarding, reinforcing, and motivational effects of nicotine. Pharmacological manipulation of this receptor deserves further consideration as a potential new valuable target for the treatment of nicotine dependence.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E (2012). The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res 1444: 45–54.
Aracil-Fernandez A, Trigo JM, Garcia-Gutierrez MS, Ortega-Alvaro A, Ternianov A, Navarro D et al (2012). Decreased cocaine motor sensitization and self-administration in mice overexpressing cannabinoid CB(2) receptors. Neuropsychopharmacology 37: 1749–1763.
Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R (2004). Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci 20: 2737–2748.
Benowitz NL (2010). Nicotine addiction. New Engl J Med 362: 2295–2303.
Benowitz NL, Fitzgerald GA, Wilson M, Zhang Q (1993). Nicotine effects on eicosanoid formation and hemostatic function: comparison of transdermal nicotine and cigarette smoking. J Am Coll Cardiol 22: 1159–1167.
Biala G, Weglinska B (2005). Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res 51: 483–488.
Burokas A, Gutierrez-Cuesta J, Martin-Garcia E, Maldonado R (2012). Operant model of frustrated expected reward in mice. Addict Biol 17: 770–782.
Castane A, Berrendero F, Maldonado R (2005). The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav 81: 381–386.
Castane A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O (2002). Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology 43: 857–867.
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C et al (2003). Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23: 7820–7829.
Cohen C, Perrault G, Voltz C, Steinberg R, Soubrie P (2002). SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol 13: 451–463.
Corrigall WA, Coen KM, Adamson KL (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res 653: 278–284.
Cossu G, Ledent C, Fattore L, Imperato A, Bohme GA, Parmentier M et al (2001). Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res 118: 61–65.
Damaj MI, Kao W, Martin BR (2003). Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther 307: 526–534.
David V, Besson M, Changeux JP, Granon S, Cazala P (2006). Reinforcing effects of nicotine microinjections into the ventral tegmental area of mice: dependence on cholinergic nicotinic and dopaminergic D1 receptors. Neuropharmacology 50: 1030–1040.
Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT (2006). Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12: 178–207.
Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M (2002). Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci 15: 1810–1818.
Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B (2012). Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One 7: e29900.
Glick SD, Sell EM, McCallum SE, Maisonneuve IM (2011). Brain regions mediating alpha3beta4 nicotinic antagonist effects of 18-MC on nicotine self-administration. Eur J Pharmacol 669: 71–75.
Hays JT, Ebbert JO (2010). Adverse effects and tolerability of medications for the treatment of tobacco use and dependence. Drugs 70: 2357–2372.
Ikemoto S, Qin M, Liu ZH (2006). Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci 26: 723–730.
Ishiguro H, Iwasaki S, Teasenfitz L, Higuchi S, Horiuchi Y, Saito T et al (2007). Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. Pharmacogenomics J 7: 380–385.
Jackson KJ, McLaughlin JP, Carroll FI, Damaj MI (2012). Effects of the kappa opioid receptor antagonist, norbinaltorphimine, on stress and drug-induced reinstatement of nicotine-conditioned place preference in mice. Psychopharmacology (Berl) 226: 763–768.
Jafari MR, Golmohammadi S, Ghiasvand F, Zarrindast MR, Djahanguiri B (2007). Influence of nicotinic receptor modulators on CB2 cannabinoid receptor agonist (JWH133)-induced antinociception in mice. Behav Pharmacol 18: 691–697.
Jones IW, Wonnacott S (2004). Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci 24: 11244–11252.
Laviolette SR, van der Kooy D (2004). The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5: 55–65.
Le Foll B, Goldberg SR (2004). Rimonabant, a CB1 antagonist, blocks nicotine-conditioned place preferences. Neuroreport 15: 2139–2143.
Liu L, Zhao-Shea R, McIntosh JM, Gardner PD, Tapper AR (2012). Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits. Mol Pharmacol 81: 541–548.
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408.
Martin-Garcia E, Barbano MF, Galeote L, Maldonado R (2009). New operant model of nicotine-seeking behaviour in mice. Int J Neuropsychopharmacol 12: 343–356.
Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI (2008). The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther 326: 483–492.
Mihalak KB, Carroll FI, Luetje CW (2006). Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70: 801–805.
Navarrete F, Perez-Ortiz JM, Manzanares J (2012). Cannabinoid CB(2) receptor-mediated regulation of impulsive-like behaviour in DBA/2 mice. Br J Pharmacol 165: 260–273.
Nisell M, Nomikos GG, Svensson TH (1994). Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16: 36–44.
Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L et al (2008a). Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One 3: e1640.
Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L et al (2008b). Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci 1139: 434–449.
Palkovits M (1983). Punch sampling biopsy technique. Methods Enzymol 103: 368–376.
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates Academic Press. Harcourt Science and Technology Company: New York.
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997). Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390: 401–404.
Plaza-Zabala A, Flores A, Maldonado R, Berrendero F (2012). Hypocretin/orexin signaling in the hypothalamic paraventricular nucleus is essential for the expression of nicotine withdrawal. Biol Psychiatry 71: 214–223.
Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM et al (2008). Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 28: 12318–12327.
Rahman S, Zhang J, Corrigall WA (2003). Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: in vivo microdialysis study. Neurosci Lett 348: 61–64.
Salas R, Pieri F, De Biasi M (2004). Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. J Neurosci 24: 10035–10039.
Soria G, Barbano MF, Maldonado R, Valverde O (2008). A reliable method to study cue-, priming-, and stress-induced reinstatement of cocaine self-administration in mice. Psychopharmacology (Berl) 199: 593–603.
Soria G, Mendizabal V, Tourino C, Robledo P, Ledent C, Parmentier M et al (2005). Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology 30: 1670–1680.
Stoker AK, Semenova S, Markou A (2008). Affective and somatic aspects of spontaneous and precipitated nicotine withdrawal in C57BL/6J and BALB/cByJ mice. Neuropharmacology 54: 1223–1232.
Stolerman IP, Jarvis MJ (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117: 2–10 Discussion 14–20.
Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R (2002). Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol 135: 564–578.
Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR et al (2011). Brain cannabinoid CB receptors modulate cocaine’s actions in mice. Nat Neurosci 14: 1160–1166.
Acknowledgements
We thank Patricia Rodríguez, Analía Rico, and Roberto Cabrera for invaluable technical assistance and collaboration.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website .
Rights and permissions
About this article
Cite this article
Navarrete, F., Rodríguez-Arias, M., Martín-García, E. et al. Role of CB2 Cannabinoid Receptors in the Rewarding, Reinforcing, and Physical Effects of Nicotine. Neuropsychopharmacol 38, 2515–2524 (2013). https://doi.org/10.1038/npp.2013.157
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.157
Keywords
This article is cited by
-
Cannabinoid receptor 2 deletion influences social memory and synaptic architecture in the hippocampus
Scientific Reports (2021)
-
Cannabinoids and the expanded endocannabinoid system in neurological disorders
Nature Reviews Neurology (2020)
-
Age-dependent hormesis-like effects of the synthetic cannabinoid CP55940 in C57BL/6 mice
npj Aging and Mechanisms of Disease (2020)
-
The impact of cannabinoid type 2 receptors (CB2Rs) in neuroprotection against neurological disorders
Acta Pharmacologica Sinica (2020)
-
Targeting the endocannabinoid system: a predictive, preventive, and personalized medicine-directed approach to the management of brain pathologies
EPMA Journal (2020)