Abstract
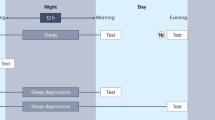
Sleep has a pivotal role in the consolidation of declarative memory. The coordinated neuronal replay of information encoded before sleep has been identified as a key process. It is assumed that the repeated reactivation of firing patterns in glutamatergic neuron assemblies translates into plastic synaptic changes underlying the formation of longer-term neuronal representations. Here, we tested the effects of blocking and enhancing glutamatergic neurotransmission during sleep on declarative memory consolidation in humans. We conducted three placebo-controlled, crossover, double-blind studies in which participants learned a word-pair association task. Afterwards, they slept in a sleep laboratory and received glutamatergic modulators. Our first two studies aimed at impairing consolidation by administering the NMDA receptor blocker ketamine and the AMPA receptor blocker caroverine during retention sleep, which, paradoxically, remained unsuccessful, inasmuch as declarative memory performance was unaffected by the treatment. However, in the third study, administration of the NMDA receptor coagonist D-cycloserine (DCS) during retention sleep facilitated consolidation of declarative memory (word pairs) but not consolidation of a procedural control task (finger sequence tapping). Administration of DCS during a wake interval remained without effect on retention of word pairs but improved encoding of numbers. From the overall pattern, we conclude that the consolidation of hippocampus-dependent declarative memory during sleep relies on NMDA-related plastic processes that differ from those processes leading to wake encoding. We speculate that glutamatergic activation during sleep is not only involved in consolidation but also in forgetting of hippocampal memory with both processes being differentially sensitive to DCS and unselective blockade of NMDA and AMPA receptors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T et al (2008). Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58: 261–272.
Antonenko D, Diekelmann S, Olsen C, Born J, Molle M (2013). Napping to renew learning capacity: enhanced encoding after stimulation of sleep slow oscillations. Eur J Neurosci 37: 1142–1151.
Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T et al (2009). Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron 61: 454–466.
Baez MV, Oberholzer MV, Cercato MC, Snitcofsky M, Aguirre AI, Jerusalinsky DA (2013). NMDA receptor subunits in the adult rat hippocampus undergo similar changes after 5 minutes in an open field and after LTP induction. PLoS One 8: e55244.
Barrett TR, Ekstrand BR (1972). Effect of sleep on memory. 3. Controlling for time-of-day effects. J Exp Psychol 96: 321–327.
Bast T, da Silva BM, Morris RG (2005). Distinct contributions of hippocampal NMDA and AMPA receptors to encoding and retrieval of one-trial place memory. J Neurosci 25: 5845–5856.
Behrens CJ, van den Boom LP, de Hoz L, Friedman A, Heinemann U (2005). Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci 8: 1560–1567.
Ben Mamou C, Gamache K, Nader K (2006). NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci 9: 1237–1239.
Billard JM, Rouaud E (2007). Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. Eur J Neurosci 25: 2260–2268.
Born J, Feld GB (2012). Sleep to upscale, sleep to downscale: balancing homeostasis and plasticity. Neuron 75: 933–935.
Chauvette S, Seigneur J, Timofeev I (2012). Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron 75: 1105–1113.
Chen HH, Liao PF, Chan MH (2011). mGluR5 positive modulators both potentiate activation and restore inhibition in NMDA receptors by PKC dependent pathway. J Biomed Sci 18: 19.
Chen Z, Duan M, Lee H, Ruan R, Ulfendahl M (2003). Pharmacokinetics of caroverine in the inner ear and its effects on cochlear function after systemic and local administrations in guinea pigs. Audiol Neurootol 8: 49–56.
Cohen J (1992). A power primer. Psychol Bull 112: 155–159.
Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G (1999). Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci 19: RC20.
Day M, Langston R, Morris RG (2003). Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature 424: 205–209.
Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G et al (2010). Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci USA 107: 17839–17844.
Diekelmann S, Born J (2010). The memory function of sleep. Nat Rev Neurosci 11: 114–126.
Diekelmann S, Buchel C, Born J, Rasch B (2011). Labile or stable: opposing consequences for memory when reactivated during waking and sleep. Nat Neurosci 14: 381–386.
Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE et al (1997). Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4-5 hours per night. Sleep 20: 267–277.
Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J (2010). The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13: 995–1002.
Espinosa F, Kavalali ET (2009). NMDA receptor activation by spontaneous glutamatergic neurotransmission. J Neurophysiol 101: 2290–2296.
Faul F, Erdfelder E, Lang AG, Buchner A (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39: 175–191.
Frank MG, Benington JH (2006). The role of sleep in memory consolidation and brain plasticity: dream or reality? Neuroscientist 12: 477–488.
Gais S, Albouy G, Boly M, Dang-Vu TT, Darsaud A, Desseilles M et al (2007). Sleep transforms the cerebral trace of declarative memories. Proc Natl Acad Sci USA 104: 18778–18783.
Gais S, Rasch B, Wagner U, Born J (2008). Visual-procedural memory consolidation during sleep blocked by glutamatergic receptor antagonists. J Neurosci 28: 5513–5518.
Hardt O, Migues PV, Hastings M, Wong J, Nader K (2010). PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus 20: 691–695.
Hardt O, Nader K, Nadel L (2013). Decay happens: the role of active forgetting in memory. Trends Cogn Sci 17: 111–120.
Hartvig P, Valtysson J, Lindner KJ, Kristensen J, Karlsten R, Gustafsson LL et al (1995). Central nervous system effects of subdissociative doses of (S)-ketamine are related to plasma and brain concentrations measured with positron emission tomography in healthy volunteers. Clin Pharmacol Ther 58: 165–173.
Herman JP, Mueller NK, Figueiredo H (2004). Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann NY Acad Sci 1018: 35–45.
Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC (1973). Quantification of sleepiness: a new approach. Psychophysiology 10: 431–436.
Hrabetova S, Sacktor TC (2001). Transient translocation of conventional protein kinase C isoforms and persistent downregulation of atypical protein kinase Mzeta in long-term depression. Brain Res Mol Brain Res 95: 146–152.
Inostroza M, Born J (2013). Sleep for preserving and transforming episodic memory. Annu Rev Neurosci 36: 79–102.
Janke W, Debus G (1978) Die Eigenschaftswörterliste EWL. Hogrefe: Göttingen, Germany.
Ji D, Wilson MA (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–107.
Kalisch R, Holt B, Petrovic P, De Martino B, Kloppel S, Buchel C et al (2009). The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cereb Cortex 19: 187–196.
Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R et al (1998). The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868.
King C, Henze DA, Leinekugel X, Buzsaki G (1999). Hebbian modification of a hippocampal population pattern in the rat. J Physiol 521 (Pt 1): 159–167.
Kochlamazashvili G, Bukalo O, Senkov O, Salmen B, Gerardy-Schahn R, Engel AK et al (2012). Restoration of synaptic plasticity and learning in young and aged NCAM-deficient mice by enhancing neurotransmission mediated by GluN2A-containing NMDA receptors. J Neurosci 32: 2263–2275.
Kuriyama K, Honma M, Koyama S, Kim Y (2011a). D-cycloserine facilitates procedural learning but not declarative learning in healthy humans: a randomized controlled trial of the effect of D-cycloserine and valproic acid on overnight properties in the performance of non-emotional memory tasks. Neurobiol Learn Mem 95: 505–509.
Kuriyama K, Honma M, Shimazaki M, Horie M, Yoshiike T, Koyama S et al (2011b). An N-methyl-D-aspartate receptor agonist facilitates sleep-independent synaptic plasticity associated with working memory capacity enhancement. Sci Rep 1: 127.
Kuriyama K, Honma M, Soshi T, Fujii T, Kim Y (2011c). Effect of D-cycloserine and valproic acid on the extinction of reinstated fear-conditioned responses and habituation of fear conditioning in healthy humans: a randomized controlled trial. Psychopharmacology (Berl) 218: 589–597.
Lang PJ, Bradley MM, Cuthbert BN (2008). International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida: Gainesville, FL.
Lee JL, Milton AL, Everitt BJ (2006). Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26: 10051–10056.
Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M et al (2004). Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science 304: 1021–1024.
Malenka RC, Bear MF (2004). LTP and LTD: an embarrassment of riches. Neuron 44: 5–21.
Malenka RC, Nicoll RA (1999). Long-term potentiation—a decade of progress? Science 285: 1870–1874.
Marshall L, Helgadottir H, Molle M, Born J (2006). Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613.
Marshall L, Molle M, Hallschmid M, Born J (2004). Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci 24: 9985–9992.
Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT et al (2010). PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci 13: 630–634.
Mölle M, Born J (2011). Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res 193: 93–110.
Nader K, Hardt O (2009). A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10: 224–234.
O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J (2010). Play it again: reactivation of waking experience and memory. Trends Neurosci 33: 220–229.
Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A et al (2010). The N-methyl-D-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry 67: 1205–1211.
Oudiette D, Paller KA (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn Sci 17: 142–149.
Plihal W, Born J (1997). Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9: 534–547.
Rasch B, Buchel C, Gais S, Born J (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315: 1426–1429.
Rechtschaffen A, Kales A (1968) A Manual of Standardized Terminology, Technique and Scoring System for Sleep Stages of Human Sleep. Brain Information Service, Brain Information Institute, UCLA: Los Angeles, CA, USA.
Ribeiro S, Shi X, Engelhard M, Zhou Y, Zhang H, Gervasoni D et al (2007). Novel experience induces persistent sleep-dependent plasticity in the cortex but not in the hippocampus. Front Neurosci 1: 43–55.
Rosenzweig ES, Barnes CA, McNaughton BL (2002). Making room for new memories. Nat Neurosci 5: 6–8.
Rudoy JD, Voss JL, Westerberg CE, Paller KA (2009). Strengthening individual memories by reactivating them during sleep. Science 326: 1079.
Sheinin A, Shavit S, Benveniste M (2001). Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 41: 151–158.
Siapas AG, Wilson MA (1998). Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21: 1123–1128.
Skaggs WE, McNaughton BL (1996). Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271: 1870–1873.
Tononi G, Cirelli C (2006). Sleep function and synaptic homeostasis. Sleep Med Rev 10: 49–62.
Van Der Werf YD, Altena E, Schoonheim MM, Sanz-Arigita EJ, Vis JC, De Rijke W et al (2009). Sleep benefits subsequent hippocampal functioning. Nat Neurosci 12: 122–123.
Villarreal DM, Do V, Haddad E, Derrick BE (2002). NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat Neurosci 5: 48–52.
Wagner U, Gais S, Born J (2001). Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem 8: 112–119.
Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R (2003). Sleep and the time course of motor skill learning. Learn Mem 10: 275–284.
Watson D, Clark LA, Tellegen A (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 54: 1063–1070.
Wilson MA, McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679.
Zhu M, Nix DE, Adam RD, Childs JM, Peloquin CA (2001). Pharmacokinetics of cycloserine under fasting conditions and with high-fat meal, orange juice, and antacids. Pharmacotherapy 21: 891–897.
Acknowledgements
We thank Felix Machleidt for medical supervision of the study; Simon Schütt, Cornelia Herzmann, and Maike Hansmann for assisting in data collection; and Martina Grohs and Heidi Ruf for performing blood analyses. This research was supported by a grant from the Deutsche Forschungsgemeinschaft SFB 654 ‘Plasticity and Sleep’.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Feld, G., Lange, T., Gais, S. et al. Sleep-Dependent Declarative Memory Consolidation—Unaffected after Blocking NMDA or AMPA Receptors but Enhanced by NMDA Coagonist D-Cycloserine. Neuropsychopharmacol 38, 2688–2697 (2013). https://doi.org/10.1038/npp.2013.179
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.179
Keywords
This article is cited by
-
Cognitive and emotional empathy after stimulation of brain mineralocorticoid and NMDA receptors in patients with major depression and healthy controls
Neuropsychopharmacology (2020)
-
Steroid hormone secretion after stimulation of mineralocorticoid and NMDA receptors and cardiovascular risk in patients with depression
Translational Psychiatry (2020)
-
Overnight memory consolidation facilitates rather than interferes with new learning of similar materials—a study probing NMDA receptors
Neuropsychopharmacology (2018)
-
Central Nervous Insulin Signaling in Sleep-Associated Memory Formation and Neuroendocrine Regulation
Neuropsychopharmacology (2016)