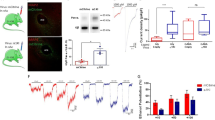
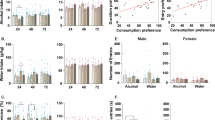
Abstract
Bilateral stereotactic lesioning of the nucleus accumbens (NAc) core reduces relapse rates in alcohol-dependent patients but may cause irreversible cognitive deficits. Deep brain stimulation has similar effects but requires costly implanted hardware and regular surgical maintenance. Therefore, there is considerable interest in refining these approaches to develop reversible, minimally invasive treatments for alcohol dependence. Toward this end, we evaluated the feasibility of a reverse pharmacogenetic approach in a preclinical mouse model. We first assessed the predictive validity of a limited access ethanol consumption paradigm by confirming that electrolytic lesions of the NAc core decreased ethanol consumption, recapitulating the effects of similar lesions in humans. We then used this paradigm to test the effect of modulating activity in the NAc using the Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) hM3Dq and hM4Di. We found that increasing activity with hM3Dq had no effect, but suppressing activity with hM4Di reduced alcohol consumption to a similar extent as lesioning without affecting consumption of water or sucrose. These results may represent early steps toward a novel neurosurgical treatment modality for alcohol dependence that is reversible and externally titratable, yet highly targetable and less invasive than current approaches such as lesioning or deep brain stimulation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA et al (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39.
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168.
Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD (2011). Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med 41: 516–524.
CDC (2008). Alcohol Related Disease Impact (ARDI) application. Available at http://apps.nccd.cdc.gov/DACH_ARDI/Default.aspx.
Chang WH, Lin SK, Lane HY, Wei FC, Hu WH, Lam YW et al (1998). Reversible metabolism of clozapine and clozapine N-oxide in schizophrenic patients. Prog Neuro-psychopharmacol Biol Psychiatry 22: 723–739.
Chaudhri N, Sahuque LL, Schairer WW, Janak PH (2010). Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology 35: 783–791.
Christine CW, Starr PA, Larson PS, Eberling JL, Jagust WJ, Hawkins RA et al (2009). Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology 73: 1662–1669.
Dhaher R, Finn DA, Oberbeck DL, Yoneyama N, Snelling CC, Wu W et al (2009). Electrolytic lesions of the medial nucleus accumbens shell selectively decrease ethanol consumption without altering preference in a limited access procedure in C57BL/6J mice. Pharmacol Biochem Behav 92: 335–342.
Dole VP, Gentry RT (1984). Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci USA 81: 3543–3546.
Fenno L, Yizhar O, Deisseroth K (2011). The development and application of optogenetics. Annu Rev Neurosci 34: 389–412.
Furay AR, Neumaier JF, Mullenix AT, Kaiyala KK, Sandygren NK, Hoplight BJ (2011). Overexpression of 5-HT(1B) mRNA in nucleus accumbens shell projection neurons differentially affects microarchitecture of initiation and maintenance of ethanol consumption. Alcohol 45: 19–32.
Gremel CM, Cunningham CL (2008). Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci 28: 1076–1084.
Hansen S, Fahlke C, Hard E, Thomasson R (1995). Effects of ibotenic acid lesions of the ventral striatum and the medial prefrontal cortex on ethanol consumption in the rat. Alcohol 12: 397–402.
He F, Guan H, Zhao Z, Miao X, Zhou Q, Li L et al (2008). Evaluation of short-term psychological functions in opiate addicts after ablating the nucleus accumbens via stereotactic surgery. Stereotact Funct Neurosurg 86: 320–329.
Heinz A, Beck A, Grusser SM, Grace AA, Wrase J (2009). Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol 14: 108–118.
Heinze HJ, Heldmann M, Voges J, Hinrichs H, Marco-Pallares J, Hopf JM et al (2009). Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci 3: 22.
Hoplight BJ, Sandygren NA, Neumaier JF (2006). Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol 38: 73–79.
Johansson AK, Hansen S (2000). Increased alcohol intake and behavioral disinhibition in rats with ventral striatal neuron loss. Physiol Behav 70: 453–463.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS et al (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428.
Kuhn J, Grundler TO, Bauer R, Huff W, Fischer AG, Lenartz D et al (2011). Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol 16: 620–623.
LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN et al (2011). AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 10: 309–319.
Lobo MK, Nestler EJ (2011). The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 5: 41.
Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E et al (2011). Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333: 637–642.
Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84: 53–63.
Rodgers DA, McClearn G (1962). Mouse strain differences in preference for various concentrations of alcohol. Q J Stud Alcohol 23: 26–33.
Rogan SC, Roth BL (2011). Remote control of neuronal signaling. Pharmacol Rev 63: 291–315.
Sjulson L, Miesenböck G (2008). Photocontrol of neural activity: biophysical mechanisms and performance in vivo. Chem Rev 108: 1588–1602.
Souweidane MM, Fraser JF, Arkin LM, Sondhi D, Hackett NR, Kaminsky SM et al (2010). Gene therapy for late infantile neuronal ceroid lipofuscinosis: neurosurgical considerations. J Neurosurg Pediatr 6: 115–122.
Tanchuck MA, Yoneyama N, Ford MM, Fretwell AM, Finn DA (2011). Assessment of GABA-B, metabotropic glutamate, and opioid receptor involvement in an animal model of binge drinking. Alcohol 45: 33–44.
Tepper JM, Tecuapetla F, Koos T, Ibanez-Sandoval O (2010). Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat 4: 150.
Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H et al (2005). Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci 77: 130–139.
Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G et al (2004). DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res 28: 720–728.
Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G et al (2001). Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem 78: 1094–1103.
Voges J, Muller U, Bogerts B, Munte T, Heinze HJ (2012). Deep brain stimulation surgery for alcohol addiction. World Neurosurg 80: e21–S28.e31.
Wu HM, Wang XL, Chang CW, Li N, Gao L, Geng N et al (2010). Preliminary findings in ablating the nucleus accumbens using stereotactic surgery for alleviating psychological dependence on alcohol. Neurosci Lett 473: 77–81.
Acknowledgements
We thank Michael Krashes, Brad Lowell, Bryan Roth, and Jürgen Wess for sharing DREADD constructs and reagents. CNO was obtained from the NIH as part of the Rapid Access to Investigative Drug Program funded by the NINDS. We also thank Michael Long and Dmitriy Aronov for advice on electrolytic lesions, Charles Hoeffer and the NYU rodent behavior core facility for help with behavioral assays, and Jens Hjerling-Leffler for valuable comments on the manuscript. This work was supported by funds from the NYU Physician Scientist Training Program (LS); the NYU Dean’s Undergraduate Research Fund (DC); NIH grants R01 MH068469, R01 MH071679, R01 MH095147, and R01 NS081297 to GF; and grant UL1 TR000038 from the National Center for Advancing Translational Sciences, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Cassataro, D., Bergfeldt, D., Malekian, C. et al. Reverse Pharmacogenetic Modulation of the Nucleus Accumbens Reduces Ethanol Consumption in a Limited Access Paradigm. Neuropsychopharmacol 39, 283–290 (2014). https://doi.org/10.1038/npp.2013.184
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.184
Keywords
This article is cited by
-
Chemogenetic selective manipulation of nucleus accumbens medium spiny neurons bidirectionally controls alcohol intake in male and female rats
Scientific Reports (2020)
-
Downregulation of M-channels in lateral habenula mediates hyperalgesia during alcohol withdrawal in rats
Scientific Reports (2019)
-
Architectural Representation of Valence in the Limbic System
Neuropsychopharmacology (2016)
-
Rat Nucleus Accumbens Core Astrocytes Modulate Reward and the Motivation to Self-Administer Ethanol after Abstinence
Neuropsychopharmacology (2014)