Abstract
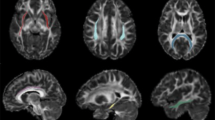
Ketamine is an N-methyl-D-aspartate (NMDA) receptor antagonist that has been found to induce schizophrenia-type symptoms in humans and is a potent and fast-acting antidepressant. It is also a relatively widespread drug of abuse, particularly in China and the UK. Acute administration has been well characterized, but the effect of extended periods of ketamine use—on brain structure in humans—remains poorly understood. We measured indices of white matter microstructural integrity and connectivity in the brain of 16 ketamine users and 16 poly-drug-using controls, and we used probabilistic tractography to quantify changes in corticosubcortical connectivity associated with ketamine use. We found a reduction in the axial diffusivity profile of white matter in a right hemisphere network of white matter regions in ketamine users compared with controls. Within the ketamine-user group, we found a significant positive association between the connectivity profile between the caudate nucleus and the lateral prefrontal cortex and dissociative experiences. These findings suggest that chronic ketamine use may be associated with widespread disruption of white matter integrity, and white matter pathways between subcortical and prefrontal cortical areas may in part predict individual differences in dissociative experiences due to ketamine use.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alexander DANIELC, Seunarine KIRANK (2010). Mathematics of Crossing Fibers.. Diffusion MRI: Theory, Methods, and Applications. Oxford University Press: New York, NY, USA. pp 451–464.
Baddeley A, Emslie H, Nimmo Smith I (1993). The spot-the-word test: a robust estimate of verbal intelligence based on lexical decision. Br J Clin Psychol 32: 55–65.
Basser PJ, Mattiello J, Lebihan D (1994). Estimation of the effective self-diffusion tensor from the Nmr Spin-Echo. J Magn Reson B 103: 247–254.
Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15: 435–455.
Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA et al (2003). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6: 750–757.
Bernstein EM, Putnam FW (1986). Development, reliability, and validity of a dissociation scale. J Nerv Ment Dis 174: 727–735.
Blakemore S-J, Choudhury S (2006). Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatr 47: 296–312.
Brozoski TJ, Brown RM, Rosvold HE, Goldman PS (1979). Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205: 929–932.
Corlett PR, Honey GD, Aitken MRF, Dickinson A, Shanks DR, Absalom AR et al (2006). Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: linking cognition, brain activity, and psychosis. Arch Gen Psychiatry 63: 611.
Corlett PR, Honey GD, Fletcher PC (2007). From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol 21: 238–252.
Dawson N, Morris BJ, Pratt JA (2011). Subanaesthetic ketamine treatment alters prefrontal cortex connectivity with thalamus and ascending subcortical systems. Schizophr Bull 39: 366–377.
Deichmann R, Schwarzbauer C, Turner R (2004). Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3T. Neuroimage 21: 757–767.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: 968–980.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K et al (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335.
Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET (2012). Abnormal brain structure implicated in stimulant drug addiction. Science 335: 601–604.
Friston KJ, Frith CD (1995). Schizophrenia: a disconnection syndrome. Clin Neurosci 3: 89–97.
Goldstein RZ, Volkow ND (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642.
Honey GD, Corlett PR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK et al (2008). Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J Neurosci 28: 6295–6303.
Jansons KM, Alexander DC (2003). Persistent angular structure: new insights from diffusion magnetic resonance imaging data. Inf Process Med Imaging 18: 672–683.
Jentsch JD, Roth RH (1999). The neuropsychopharmacology of phencyclidine: from NMDA receptor hypofunction to the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 20: 201–225.
Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD et al (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51: 199.
Laruelle M, Frankle WG, Narendran R, Kegeles LS, Adi-Dargham A (2005). Mechanism of action of antipsychotic drugs: from dopamine D(2) receptor antagonism to glutamate NMDA facilitation. Clin Ther 27: S16–S24.
Liao Y, Tang J, Corlett PR, Wang X, Yang M, Chen H et al (2011). Reduced dorsal prefrontal gray matter after chronic ketamine use’. Biol Psychiatry 69: 42–48.
Liao Y, Tang J, Ma M, Wu Z, Yang M, Wang X et al (2010). Frontal white matter abnormalities following chronic ketamine use: a diffusion tensor imaging study. Brain 133: 2115.
Lipska BK, Weinberger DR, Swerdlow NR, Geyer MA, Braff DL, Jaskiw GE (1995). Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology 122: 35–43.
Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL et al (2011). Investigation of anatomical thalamo-cortical connectivity and fmri activation in schizophrenia. Neuropsychopharmacology 37: 499–507.
Mason O, Claridge G, Jackson M (1995). New scales for the assessment of schizotypy. Pers Individ Dif 18: 7–13.
Mcdonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP (1998). Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med 4: 291–297.
Meyer F, Louilot A (2012). Early prefrontal functional blockade in rats results in schizophrenia-related anomalies in behavior and dopamine. Neuropsychopharmacology 37: 2233–2243.
Moghaddam B, Adams B, Verma A, Daly D (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927.
Morgan CJA, Monaghan L, Curran HV (2004a). Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction 99: 1450–1461.
Morgan CJA, Mofeez A, Brandner B, Bromley L, Curran HV (2004b). Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology 29: 208–218.
Morgan CJA, Muetzelfeldt L, Curran HV (2010). Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction 105: 121–133.
Mori S, Wakana S, Van Zijl PCM, Nagae-Poetscher LM (2005) MRI Atlas of Human White Matter. Elsevier: Amsterdam, The Netherlands.
Narendran R, Frankle WG, Keefe R, Gil R, Martinez D, Slifstein M et al (2005). Altered prefrontal dopaminergic function in chronic recreational ketamine users. Am J Psychiatry 162: 2352–2359.
Nichols T. E, Holmes A. P (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25.
Oouchi H, Yamada K, Sakai K, Kizu O, Kubota T, Ito H et al (2007). Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. Am J Neuroradiol 28: 1102–1106.
Pitt David, Gonzales E, Cross AH, Goldberg MP (2010). Dysmyelinated axons in shiverer mice are highly vulnerable to A-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor-mediated toxicity. Brain Res 1309: 146–154.
Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003). Reduction of Eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med 49: 177–182.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505.
Smith S. M (2002). Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H et al (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: 208–219.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld A. H (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722.
Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH et al (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26: 132–140.
Verma A, Moghaddam B (1996). NMDA receptor antagonists impair prefrontal cortex function as accessed via spatial delayed alternation performance in rats: modulation by dopamine. J Neurosci 16: 373–379.
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL et al (2007). Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36: 630–644.
Wheeler-Kingshott CAM, Hickman SJ, Parker GJM, Ciccarelli O, Symms MR, Miller DH et al (2002). Investigating cervical spinal cord structure using axial diffusion tensor imaging. Neuroimage 16: 93–102.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Edward Roberts, R., Curran, H., Friston, K. et al. Abnormalities in White Matter Microstructure Associated with Chronic Ketamine Use. Neuropsychopharmacol 39, 329–338 (2014). https://doi.org/10.1038/npp.2013.195
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.195
Keywords
This article is cited by
-
Aberrant sense of agency induced by delayed prediction signals in schizophrenia: a computational modeling study
Schizophrenia (2023)
-
Chronic administration of ketamine induces cognitive deterioration by restraining synaptic signaling
Molecular Psychiatry (2021)
-
Effects of early ketamine exposure on cerebral gray matter volume and functional connectivity
Scientific Reports (2020)
-
Neurocognitive Effects of Ketamine and Association with Antidepressant Response in Individuals with Treatment-Resistant Depression: A Randomized Controlled Trial
Neuropsychopharmacology (2015)