Abstract
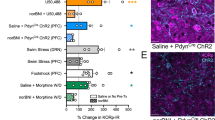
Pain is often associated with depression of behavior and mood, and relief of pain-related depression is a common goal of treatment. This study tested the hypothesis that pain-related behavioral depression is mediated by activation of endogenous κ-opioid systems and subsequent depression of mesolimbic dopamine release. Adult male Sprague–Dawley rats were implanted with electrodes targeting the medial forebrain bundle (for behavior studies of intracranial self-stimulation (ICSS)) or with cannulae for microdialysis measures of nucleus accumbens dopamine (NAc DA). Changes in ICSS and NAc DA were examined after treatment with a visceral noxious stimulus (intraperitoneal injection of dilute lactic acid) or an exogenous κ-agonist (U69593). Additional studies examined the sensitivity of acid and U69593 effects to blockade by two analgesics (the nonsteroidal antiinflammatory drug ketoprofen and the μ-opioid agonist morphine) or by the κ-antagonist norbinaltorphimine (norBNI). The effects of acid were also examined on mRNA expression for prodynorphin (PDYN) and κ-opioid receptors (KORs) in mesocorticolimbic brain regions. Both acid and U69593 depressed ICSS and extracellular levels of NAc DA. Pain-related acid effects were blocked by ketoprofen and morphine but not by norBNI. The U69593 effects were blocked by norBNI but not by ketoprofen, and were only attenuated by morphine. Acid did not significantly alter PDYN or KOR in NAc, but it produced a delayed increase in PDYN in prefrontal cortex. These results support a key role for the mesolimbic DA system, but a more nuanced role for endogenous κ-opioid systems, in mediating acute pain-related behavioral depression in rats.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Altarifi AA, Negus SS (2011). Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 22: 663–673.
Amato D, Natesan S, Yavich L, Kapur S, Muller CP (2011). Dynamic regulation of dopamine and serotonin responses to salient stimuli during chronic haloperidol treatment. Int J Neuropsychopharmacol 14: 1327–1339.
Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S et al (2012). Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain 16: 485–495.
Bair MJ, Robinson RL, Katon W, Kroenke K (2003). Depression and pain comorbidity: a literature review. Arch Intern Med 163: 2433–2445.
Bauer CT, Banks ML, Blough BE, Negus SS (2013). Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168: 850–862.
Baumann MH, Char GU, De Costa BR, Rice KC, Rothman RB (1994). Gbr12909 attenuates cocaine-induced activation of mesolimbic dopamine neurons in the rat. J Pharmacol Exp Ther 271: 1216–1222.
Becerra L, Borsook D (2008). Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain 12: 866–869.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ et al (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–868.
Borsook D, Becerra L, Carlezon WA Jr, Shaw M, Renshaw P, Elman I et al (2007). Reward-aversion circuitry in analgesia and pain: implications for psychiatric disorders. Eur J Pain 11: 7–20.
Bray GE, Ying Z, Baillie LD, Zhai R, Mulligan SJ, Verge VM (2013). Extracellular ph and neuronal depolarization serve as dynamic switches to rapidly mobilize trka to the membrane of adult sensory neurons. J Neurosci 33: 8202–8215.
Bruchas MR, Land BB, Chavkin C (2010). The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res 1314: 44–55.
Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S et al (2007). Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-jun n-terminal kinase. J Biol Chem 282: 29803–29811.
Cadoni C, DiChiara G (2007). Differences in dopamine responsiveness of drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. J Neurochem 103: 487–499.
Carlezon WA Jr., Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS et al (2006). Depressive-like effects of the kappa-opioid receptor agonist salvinorin a on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316: 440–447.
Carlezon WA Jr, Chartoff EH (2007). Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2: 2987–2995.
Chartoff EH, Papadopoulou M, MacDonald ML, Parsegian A, Potter D, Konradi C et al (2009). Desipramine reduces stress-activated dynorphin expression and creb phosphorylation in NAc tissue. Mol Pharmacol 75: 704–712.
Chavkin C, James IF, Goldstein A (1982). Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science 215: 413–415.
Cleeland CS, Ryan KM (1994). Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore 23: 129–138.
Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ (2012). Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153: 876–884.
De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL et al (2013). Capturing the aversive state of cephalic pain preclinically. Ann Neurol (e-pub ahead of print, doi:10.1002/ana.23922).
Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S et al (2013). Acid-sensing ion channels (ASICS): pharmacology and implication in pain. Pharmacol Ther 128: 549–558.
Dharmshaktu P, Tayal V, Kalra BS (2012). Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol 52: 6–17.
Di Chiara G, Imperato A (1988a). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Di Chiara G, Imperato A (1988b). Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244: 1067–1080.
Endoh T, Matsuura H, Tanaka C, Nagase H (1992). Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch Int Pharmacodyn Ther 316: 30–42.
Jarcho JM, Mayer EA, Jiang ZK, Feier NA, London ED (2012). Pain, affective symptoms, and cognitive deficits in patients with cerebral dopamine dysfunction. Pain 153: 744–754.
Kalivas PW, Duffy P (1995). Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675: 325–328.
Knoll AT, Carlezon WA Jr. (2010). Dynorphin, stress, and depression. Brain Res 1314: 56–73.
Kwilasz AJ, Negus SS (2012). Dissociable effects of the cannabinoid receptor agonists {delta}9-tetrahydrocannabinol and CP55940 on pain-stimulated versus pain-depressed behavior in rats. J Pharmacol Exp Ther 343: 389–400.
Marinelli S, Pascucci T, Bernardi G, Puglisi-Allegra S, Mercuri NB (2005). Activation of TRPV1 in the VTA excites dopaminergic neurons and increases chemical- and noxious-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology 30: 864–870.
Martin TJ, Buechler NL, Kahn W, Crews JC, Eisenach JC (2004). Effects of laparotomy on spontaneous exploratory activity and conditioned operant responding in the rat: a model for postoperative pain. Anesthesiology 101: 191–203.
McLaughlin JP, Marton-Popovici M, Chavkin C (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci 23: 5674–5683.
Muschamp JW, Van’t Veer A, Parsegian A, Gallo MS, Chen M, Neve RL et al (2011). Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci 31: 3095–3103.
Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S et al (2013). Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci USA 109: 20709–20713.
Negus SS (2013). Expression and treatment of pain-related behavioral depression. Lab Animal 42: 292–300.
Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW (2010a). Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. In: Szallasi A (ed) Methods in Molecular Biology: Analgesia. Humana Press: New York. pp 79–91.
Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC (2010b). Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210: 149–159.
Negus SS, O'Connell R, Morrissey E, Cheng K, Rice KC (2012). Effects of peripherally restricted kappa opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340: 501–509.
Olds J, Milner P (1954). Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47: 419–427.
Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS (2009). Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144: 170–177.
Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA Jr (2001). Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated camp response element-binding protein expression in nucleus accumbens. J Neurosci 21: 7397–7403.
Schad CA, Justice JB Jr., Holtzman SG (1995). Naloxone reduces the neurochemical and behavioral effects of amphetamine but not those of cocaine. Eur J Pharmacol 275: 9–16.
Stellar JR, Stellar E (1985) The Neurobiology of Motivation and Reward. Springer-Verlag: New York.
Stevenson GW, Bilsky EJ, Negus SS (2006). Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in c57bl/6j mice. J Pain 7: 408–416.
Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS et al (2009). Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci 85: 309–315.
Tejeda HA, Counotte DS, Oh E, Ramamoorthy S, Schultz-Kuszak KN, Bäckman CM et al (2013). Prefrontal cortical kappa-opioid receptor modulation of local neurotransmission and conditioned place aversion. Neuropsychopharmacology 38: 1770–1779.
Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA Jr. (2004). Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172: 463–470.
Wee S, Koob GF (2010). The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 210: 121–135.
Wise RA (2008). Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14: 169–183.
Wood PB (2008). Role of central dopamine in pain and analgesia. Expert Rev Neurother 8: 781–797.
Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ (2005). Effects of the plant-derived hallucinogen salvinorin a on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 179: 551–558.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Leitl, M., Onvani, S., Bowers, M. et al. Pain-Related Depression of the Mesolimbic Dopamine System in Rats: Expression, Blockade by Analgesics, and Role of Endogenous κ-opioids. Neuropsychopharmacol 39, 614–624 (2014). https://doi.org/10.1038/npp.2013.236
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.236
Keywords
This article is cited by
-
Acute pain-related depression of operant responding maintained by social interaction or food in male and female rats
Psychopharmacology (2022)
-
Pain-induced impulsivity is sexually dimorphic and mu-opioid receptor sensitive in rats
Psychopharmacology (2021)
-
Sex-dependent neurobiological features of prenatal immune activation via TLR7
Molecular Psychiatry (2020)
-
Lappaconitine, a C18-diterpenoid alkaloid, exhibits antihypersensitivity in chronic pain through stimulation of spinal dynorphin A expression
Psychopharmacology (2018)
-
Dissociable effects of the kappa opioid receptor agonist nalfurafine on pain/itch-stimulated and pain/itch-depressed behaviors in male rats
Psychopharmacology (2018)