Abstract
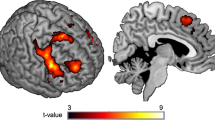
Behavioral studies have shown an alcohol-approach bias in alcohol-dependent patients: the automatic tendency to faster approach than avoid alcohol compared with neutral cues, which has been associated with craving and relapse. Although this is a well-studied psychological phenomenon, little is known about the brain processes underlying automatic action tendencies in addiction. We examined 20 alcohol-dependent patients and 17 healthy controls with functional magnetic resonance imaging (fMRI), while performing an implicit approach-avoidance task. Participants pushed and pulled pictorial cues of alcohol and soft-drink beverages, according to a content-irrelevant feature of the cue (landscape/portrait). The critical fMRI contrast regarding the alcohol-approach bias was defined as (approach alcohol>avoid alcohol)>(approach soft drink>avoid soft drink). This was reversed for the avoid-alcohol contrast: (avoid alcohol>approach alcohol)>(avoid soft drink>approach soft drink). In comparison with healthy controls, alcohol-dependent patients had stronger behavioral approach tendencies for alcohol cues than for soft-drink cues. In the approach, alcohol fMRI contrast patients showed larger blood-oxygen-level-dependent responses in the nucleus accumbens and medial prefrontal cortex, regions involved in reward and motivational processing. In alcohol-dependent patients, alcohol-craving scores were positively correlated with activity in the amygdala for the approach-alcohol contrast. The dorsolateral prefrontal cortex was not activated in the avoid-alcohol contrast in patients vs controls. Our data suggest that brain regions that have a key role in reward and motivation are associated with the automatic alcohol-approach bias in alcohol-dependent patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abrahao KP, Quadros IM, Souza-Formigoni ML (2011). Nucleus accumbens dopamine D(1) receptors regulate the expression of ethanol-induced behavioural sensitization. Int J Neuropsychopharmacol 14: 175–185.
Baler RD, Volkow ND (2006). Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med 12: 559–566.
Bechara A (2005). Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8: 1458–1463.
Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN et al (2012). Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Arch Gen Psychiatry 69: 842–852.
Brainard DH (1997). The Psychophysics Toolbox. Spat Vis 10: 433–436.
Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H et al (2001). Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm 108: 887–894.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW (2012). Approach-bias predicts development of cannabis problem severity in heavy cannabis users: results from a prospective FMRI study. PloS One 7: e42394.
Cousijn J, Goudriaan AE, Wiers RW (2011). Reaching out towards cannabis: approach-bias in heavy cannabis users predicts changes in cannabis use. Addiction 106: 1667–1674.
Deichmann R, Gottfried JA, Hutton C, Turner R (2003). Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage 19 (2 Pt 1): 430–441.
Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J (2012). Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci.
Ernst LH, Plichta MM, Dresler T, Zesewitz AK, Tupak SV, Haeussinger FB et al (2012). Prefrontal correlates of approach preferences for alcohol stimuli in alcohol dependence. Addict Biol (e-pub ahead of print; doi:10.1111/adb.12005).
Gladwin TE, Figner B, Crone EA, Wiers RW (2011). Addiction, adolescence, and the integration of control and motivation. Dev Cogn Neurosci 1: 364–376.
Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M et al (2004). Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology 175: 296–302.
Hare TA, Camerer CF, Rangel A (2009). Self-control in decision-making involves modulation of the vmPFC valuation system. Science 324: 646–648.
Hayashi T, Ko JH, Strafella AP, Dagher A (2013). Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc Natl Acad Sci USA 110: 4422–4427.
Heinz A, Beck A, Grusser SM, Grace AA, Wrase J (2009). Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol 14: 108–118.
Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM et al (2004). Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161: 1783–1789.
Hyman SE, Malenka RC (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2: 695–703.
Hyman SE, Malenka RC, Nestler EJ (2006). Neural mechanisms of addiction: the role of reward-related learning and memory. Ann Rev Neurosci 29: 565–598.
Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH (2002). Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magn Reson Imaging 20: 141–145.
Janes AC, Pizzagalli DA, Richardt S, Frederick Bde B, Holmes AJ, Sousa J et al (2010). Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology 35: 2339–2345.
Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146: 373–390.
Kahnt T, Heinzle J, Park SQ, Haynes JD (2010). The neural code of reward anticipation in human orbitofrontal cortex. Proc Natl Acad Sci USA 107: 6010–6015.
Kaufman AS, Lichtenberger E (2006) Assessing Adolescent and Adult Intelligence 3rd edn Wiley: Hoboken, NJ. pp 7.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E (2010). Everything you never wanted to know about circular analysis, but were afraid to ask. J Cereb Blood Flow Metab 30: 1551–1557.
Love A, James D, Willner P (1998). A comparison of two alcohol craving questionnaires. Addiction 93: 1091–1102.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19: 1233–1239.
Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL (2000). Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage 11 (6 Pt 1): 735–759.
Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J et al (2010). Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci 30: 7749–7753.
Park SQ, Kahnt T, Rieskamp J, Heekeren HR (2011). Neurobiology of value integration: when value impacts valuation. J Neurosci 31: 9307–9314.
Rinck M, Becker ES (2007). Approach and avoidance in fear of spiders. J Behav Therapy Exp Psychiatry 38: 105–120.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Robinson TE, Berridge KC (2003). Addiction. Annu Rev Psychol 54: 25–53.
Roelofs K, Minelli A, Mars RB, van Peer J, Toni I (2009). On the neural control of social emotional behavior. Social Cogn Affective Neurosci 4: 50–58.
Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 88: 791–804.
Schacht JP, Anton RF, Myrick H (2013). Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18: 121–133.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 (Suppl 20): 22–33 quiz 34–57.
Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 22: 158–165.
Skinner HA, Allen BA (1982). Alcohol dependence syndrome: measurement and validation. J Abnormal Psychol 91: 199–209.
Skinner HA, Sheu WJ (1982). Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Studies Alcohol 43: 1157–1170.
Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA (1983) Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA.
Stacy AW, Wiers RW (2010). Implicit cognition and addiction: a tool for explaining paradoxical behavior. Ann Rev Clin Psychol 6: 551–575.
Volkow ND, Fowler JS, Wang GJ (2004). The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology 47 (Suppl 1): 3–13.
Vollstadt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C et al (2012). Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addiction Biol 17: 807–816.
Volman I, Toni I, Verhagen L, Roelofs K (2011). Endogenous testosterone modulates prefrontal-amygdala connectivity during social emotional behavior. Cereb Cortex 21: 2282–2290.
Wiers CE, Kühn S, Javadi AH, Korucuoglu O, Wiers RW, Walter H et al (2013). Automatic approach bias towards smoking cues is present in smokers but not in ex-smokers. Psychopharmacology 229: 187–197.
Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J (2011). Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci 22: 490–497.
Wrase J, Grusser SM, Klein S, Diener C, Hermann D, Flor H et al (2002). Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry 17: 287–291.
Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T et al (2007). Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. NeuroImage 35: 787–794.
Zhou Y, Li X, Zhang M, Zhang F, Zhu C, Shen M (2012). Behavioural approach tendencies to heroin-related stimuli in abstinent heroin abusers. Psychopharmacology 221: 171–176.
Acknowledgements
We thank Ulrike Malecki, Steffen Pawelczack, Silvia Hoffman, Natalie Becht, and Jana Paeplow for the recruitment of patients from the Salus Clinic Lindow; Heiner Stuke, Amir Javadi, Sven Hädel, Steffen Weissmann, and Scott Stensland for technical support; Nick White, Thomas Gladwin, and Georgina Torbet for proofreading; and Thomas Gladwin for final comments on the manuscript. We thank the Berlin School of Mind and Brain for reimbursement of the joystick.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Wiers, C., Stelzel, C., Park, S. et al. Neural Correlates of Alcohol-Approach Bias in Alcohol Addiction: the Spirit is Willing but the Flesh is Weak for Spirits. Neuropsychopharmacol 39, 688–697 (2014). https://doi.org/10.1038/npp.2013.252
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.252
Keywords
This article is cited by
-
Acute depletion of dopamine precursors in the human brain: effects on functional connectivity and alcohol attentional bias
Neuropsychopharmacology (2021)
-
Instrumental and Pavlovian Mechanisms in Alcohol Use Disorder
Current Addiction Reports (2021)
-
Conscious and unconscious brain responses to food and cocaine cues
Brain Imaging and Behavior (2021)
-
A randomized controlled trial of a virtual reality based, approach-avoidance training program for alcohol use disorder: a study protocol
BMC Psychiatry (2020)
-
Event-related and readiness potentials when preparing to approach and avoid alcohol cues following cue avoidance training in heavy drinkers
Psychopharmacology (2020)