Abstract
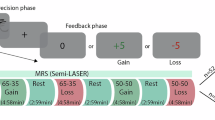
The perception of aversive stimuli is essential for human survival and depends largely on environmental context. Although aversive brain processing has been shown to involve the sensorimotor cortex, the neural and biochemical mechanisms underlying the interaction between two independent aversive cues are unclear. Based on previous work indicating ventromedial prefrontal cortex (vmPFC) involvement in the mediation of context-dependent emotional effects, we hypothesized a central role for the vmPFC in modulating sensorimotor cortex activity using a GABAergic mechanism during an aversive–aversive stimulus interaction. This approach revealed differential activations within the aversion-related network (eg, sensorimotor cortex, midcingulate, and insula) for the aversive–aversive, when compared with the aversive–neutral, interaction. Individual differences in sensorimotor cortex signal changes during the aversive–aversive interaction were predicted by GABAA receptors in both vmPFC and sensorimotor cortex. Together, these results demonstrate the central role of GABA in mediating context-dependent effects in aversion-related processing.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Amemori K, Graybiel AM (2012). Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci 15: 776–785.
Bach DR, Flandin G, Friston KJ, Dolan RJ (2010). Modelling event-related skin conductance responses. Int J Psychophysiol 75: 349–356.
Bach DR, Flandin G, Friston KJ, Dolan RJ (2009). Time-series analysis for rapid event-related skin conductance responses. J Neurosci Methods 184: 224–234.
Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ et al (2012). Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15: 1117–1119.
Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C (2003). Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. Neuroimage 18: 740–748.
Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M et al (2008). Neural response to self- and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry 65: 1176–1184.
Canbeyli R (2010). Sensorimotor modulation of mood and depression: an integrative review. Behav Brain Res 207: 249–264.
Capaday C, Richardson MP, Rothwell JC, Brooks DJ (2000). Long-term changes of GABAergic function in the sensorimotor cortex of amputees. A combined magnetic stimulation and 11C-flumazenil PET study. Exp Brain Res 133: 552–556.
Carlezon WA, Thomas MJ (2009). Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56 (Suppl 1): 122–132.
Chan T, Kyere K, Davis BR, Shemyakin A, Kabitzke PA, Shair HN et al (2011). The role of the medial prefrontal cortex in innate fear regulation in infants, juveniles, and adolescents. J Neurosci 31: 4991–4999.
Chen Z, Silva AC, Yang J, Shen J (2005). Elevated endogenous GABA level correlates with decreased fMRI signals in the rat brain during acute inhibition of GABA transaminase. J Neurosci Res 79: 383–391.
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I et al (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468: 277–282.
de Visser L, Baars AM, van 't Klooster J, van den Bos R (2011). Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male wistar rats. Front Neurosci 5: 102.
Delgado MR, Jou RL, Phelps EA (2011). Neural systems underlying aversive conditioning in humans with primary and secondary reinforcers. Front Neurosci 5: 71.
Downar J, Mikulis DJ, Davis KD (2003). Neural correlates of the prolonged salience of painful stimulation. Neuroimage 20: 1540–1551.
Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR, Raichle ME (1995). Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 373: 249–252.
Duncan NW, Enzi B, Wiebking C, Northoff G (2011). Involvement of glutamate in rest-stimulus interaction between perigenual and supragenual anterior cingulate cortex: a combined fMRI-MRS study. Hum Brain Mapp 32: 2172–2182.
Dunsmoor JE, Bandettini PA, Knight DC (2008). Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage 40: 811–817.
Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S (2007). Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp 28: 409–423.
Enomoto T, Tse MT, Floresco SB (2011). Reducing prefrontal gamma-aminobutyric acid activity induces cognitive, behavioral, and dopaminergic abnormalities that resemble schizophrenia. Biol Psychiatry 69: 432–441.
Fiorelli R, Rudolph U, Straub CJ, Feldon J, Yee BK (2008). Affective and cognitive effects of global deletion of alpha3-containing gamma-aminobutyric acid-A receptors. Behav Pharmacol 19: 582–596.
Frey KA, Holthoff VA, Koeppe RA, Jewett DM, Kilbourn MR, Kuhl DE (1991). Parametric in vivo imaging of benzodiazepine receptor distribution in human brain. Ann Neurol 30: 663–672.
Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M et al (2009). Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology 34: 932–843.
Grupe DW, Oathes DJ, Nitschke JB (2012). Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex (e-pub ahead of print) doi:10.1093/cercor/bhs175.
Hayes DJ, Hoang J, Greenshaw AJ (2011). The role of nucleus accumbens shell GABA receptors on ventral tegmental area intracranial self-stimulation and a potential role for the 5-HT2C receptor. J Psychopharmacol 25: 1661–1675.
Hayes DJ, Huxtable AG (2012). Interpreting deactivations in neuroimaging. Front Psychol 3: 27.
Hayes DJ, Northoff G (2012). Common brain activations for painful and nonpainful aversive stimuli. BMC Neurosci 13: 60.
Hayes DJ, Northoff G (2011). Identifying a network of brain regions involved in aversion-related processing: a cross-species translational investigation. Front Integr Neurosci 5: 49.
Ji G, Neugebauer V (2011). Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABA(A) receptors. J Neurophysiol 106: 2642–2652.
Johnson LR, Hou M, Prager EM, Ledoux JE (2011). Regulation of the Fear Network by Mediators of Stress: Norepinephrine Alters the Balance between Cortical and Subcortical Afferent Excitation of the Lateral Amygdala. Front Behav Neurosci 5: 23.
Klingner CM, Huonker R, Flemming S, Hasler C, Brodoehl S, Preul C et al (2011). Functional deactivations: multiple ipsilateral brain areas engaged in the processing of somatosensory information. Hum Brain Mapp 32: 127–140.
Knight DC, Waters NS, King MK, Bandettini PA (2009). Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage 49: 843–848.
Kringelbach ML, Berridge KC (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci 13: 479–487.
Kupers R, Danielsen ER, Kehlet H, Christensen R, Thomsen C (2009). Painful tonic heat stimulation induces GABA accumulation in the prefrontal cortex in man. Pain 142: 89–93.
Lehner M, Taracha E, Skorzewska A, Turzynska D, Sobolewska A, Maciejak P et al (2008). Expression of c-Fos and CRF in the brains of rats differing in the strength of a fear response. Behav Brain Res 188: 154–167.
Lehner M, Wislowska-Stanek A, Skorzewska A, Maciejak P, Szyndler J, Turzynska D et al (2010). Differences in the density of GABA-A receptor alpha-2 subunits and gephyrin in brain structures of rats selected for low and high anxiety in basal and fear-stimulated conditions, in a model of contextual fear conditioning. Neurobiol Learn Mem 94: 499–508.
Miyauchi T, Dworkin SI, Co C, Smith JE (1988). Specific effects of punishment on amino acids turnover in discrete rat brain regions. Pharmacol Biochem Behav 31: 523–531.
Montoya P, Sitges C (2006). Affective modulation of somatosensory-evoked potentials elicited by tactile stimulation. Brain Res 1068: 205–212.
Montoya P, Sitges C, Garcia-Herrera M, Izquierdo R, Truyols M, Blay N et al (2005). Abnormal affective modulation of somatosensory brain processing among patients with fibromyalgia. Psychosom Med 67: 957–963.
Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD (2011). A multisensory investigation of the functional significance of the ‘pain matrix’. Neuroimage 54: 2237–2249.
Muthukumaraswamy SD, Evans CJ, Edden RA, Wise RG, Singh KD (2011). Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum Brain Mapp 33: 455–465.
Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ (2006). Functional neuroanatomy of aversion and its anticipation. Neuroimage 29: 106–116.
Northoff G, Hayes DJ (2011). Is our self nothing but reward? Biol Psychiatry 69: 1019–1025.
Northoff G, Qin P, Nakao T (2010). Rest-stimulus interaction in the brain: a review. Trends Neurosci 33: 277–284.
Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A et al (2007). GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci 10: 1515–1517.
Onoda K, Okamoto Y, Toki S, Ueda K, Shishida K, Kinoshita A et al (2008). Anterior cingulate cortex modulates preparatory activation during certain anticipation of negative picture. Neuropsychologia 46: 102–110.
Petrini EM, Nieus T, Ravasenga T, Succol F, Guazzi S, Benfenati F et al (2011). Influence of GABAAR monoliganded states on GABAergic responses. J Neurosci 31: 1752–1761.
Piche M, Arsenault M, Rainville P (2010). Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. Pain 149: 453–462.
Qin P, Northoff G (2011). How is our self related to midline regions and the default-mode network? Neuroimage 57: 1221–1233.
Quirk GJ, Likhtik E, Pelletier JG, Pare D (2003). Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci 23: 8800–8807.
Roy M, Shohamy D, Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 16: 147–156.
Rudolph U, Knoflach F (2011). Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10: 685–697.
Salmi E, Aalto S, Hirvonen J, Langsjo JW, Maksimow AT, Oikonen V et al (2008). Measurement of GABAA receptor binding in vivo with [11C]flumazenil: a test-retest study in healthy subjects. NeuroImage 41: 260–269.
Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H et al (2000). Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci 20: 7438–7445.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356.
Smallwood J, Fitzgerald A, Miles LK, Phillips LH (2009). Shifting moods, wandering minds: negative moods lead the mind to wander. Emotion 9: 271–276.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 (Suppl 1): S208–S219.
Spreng RN (2012). The fallacy of a ‘task-negative’ network. Front Psychol 3: 145.
Steiger JH (1980). Tests for comparing elements of a correlation matrix. Psychological Bulletin 87: 245–251.
Thompson BM, Baratta MV, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF (2010). Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn Mem 17: 591–599.
Van den Stock J, Tamietto M, Sorger B, Pichon S, Grezes J, de Gelder B (2011). Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proc Natl Acad Sci USA 108: 16188–16193.
Wiebking C, Duncan NW, Qin P, Hayes DJ, Lyttelton O, Gravel P et al (2012). External awareness and GABA-A multimodal imaging study combining fMRI and [(18) F]flumazenil-PET. Hum Brain Mapp (e-pub ahead of print) doi:10.1002/hbm.22166.
Williams EJ (1959). The comparison of regression variables. J R Stat Soc B 21: 396–399.
Acknowledgements
We thank Andrea Perna and Katarina Dedovic for their help with subject recruitment, and the staff at the UNF and MNI for their skillful assistance. Also, we would like to thank Étienne Vachon-Presseau for his technical expertise regarding electrical stimulation and Oliver Lyttelton for his assistance with anatomical image acquisition. The work was supported by grants to GN from the Canadian Institutes of Health Research (CIHR), the Michael Smith Foundation, and Hope for Depression research foundation (HDRF/ISAN). DJH was funded through a CIHR Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Hayes, D., Duncan, N., Wiebking, C. et al. GABAA Receptors Predict Aversion-Related Brain Responses: An fMRI-PET Investigation in Healthy Humans. Neuropsychopharmacol 38, 1438–1450 (2013). https://doi.org/10.1038/npp.2013.40
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.40