Abstract
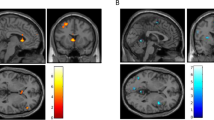
Drug-related cues are potent triggers for relapse in people with cocaine dependence. Dopamine (DA) release within a limbic network of striatum, amygdala and hippocampus has been implicated in animal studies, but in humans it has only been possible to measure effects in the striatum. The objective here was to measure drug cue-induced DA release in the amygdala and hippocampus using high-resolution PET with [18F]fallypride. Twelve cocaine-dependent volunteers (mean age: 39.6±8.0 years; years of cocaine use: 15.9±7.4) underwent two [18F]fallypride high-resolution research tomography–PET scans, one with exposure to neutral cues and one with cocaine cues. [18F]Fallypride non-displaceable-binding potential (BPND) values were derived for five regions of interest (ROI; amygdala, hippocampus, ventral limbic striatum, associative striatum, and sensorimotor striatum). Subjective responses to the cues were measured with visual analog scales and grouped using principal component analysis. Drug cue exposure significantly decreased BPND values in all five ROI in subjects who had a high-, but not low-, craving response (limbic striatum: p=0.019, associative striatum: p=0.008, sensorimotor striatum: p=0.004, amygdala: p=0.040, and right hippocampus: p=0.025). Individual differences in the cue-induced craving response predicted the magnitude of [18F]fallypride responses within the striatum (ventral limbic: r=0.581, p=0.048; associative: r=0.589, p=0.044; sensorimotor: r=0.675, p=0.016). To our knowledge this study provides the first evidence of drug cue-induced DA release in the amygdala and hippocampus in humans. The preferential induction of DA release among high-craving responders suggests that these aspects of the limbic reward network might contribute to drug-seeking behavior.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE (2006). Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron 50: 507–517.
Adolphs R, Tranel D, Damasio H, Damasio AR (1995). Fear and the human amygdala. J Neurosci 15: 5879–5891.
Alleweireldt A, Weber S, Kirschner K, Bullock B, Neisewander J (2002). Blockade or stimulation of D1 dopamine receptors attenuates cue reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacol 159: 284–293.
American Psychiatric Association (2000) Desk reference to the diagnostic criteria from DSM-IV-TR. Amer Psychiatric Pub Inc.
Becker JB (2009). Sexual differentiation of motivation: A novel mechanism? Horm Behav 55: 646–654.
Berger SP, Reid MS, Delucchi K, Hall S, Hall S, Berger SP et al (1996). Haloperidol antagonism of cue-elicited cocaine craving. Lancet 347: 504–508.
Berglind WJ, Case JM, Parker MP, Fuchs RA, See RE (2006). Dopamine D1 or D2 receptor antagonism within the basolateral amygdala differentially alters the acquisition of cocaine-cue associations necessary for cue-induced reinstatement of cocaine-seeking. Neuroscience 137: 99–706.
Berridge K (2007). The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191: 391–431.
Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M et al (2007). Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci 27: 3998–4003.
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R et al (2010a). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci 13: 419–421.
Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM et al (2010b). Dopaminergic network differences in human impulsivity. Science 329: 532.
Buffalari D, See R (2010). Amygdala mechanisms of Pavlovian psychostimulant conditioning and relapse. Behav Neurosci Drug Addict 3: 73–99.
Ceccarini J, Vrieze E, Koole M, Muylle T, Bormans G, Claes S et al (2012). Optimized in vivo detection of dopamine release using 18F-Fallypride PET. J Nuclear Med 53: 1565–1572.
Chase HW, Eickhoff SB, Laird AR, Hogarth L (2011). The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry 70: 785–793.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP (2010). Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend 111: 58–63.
Cunningham VJ, Pike VW, Bailey D, Freemantle CA, Page BC, Jones AK et al (1991). A method of studying pharmacokinetics in man at picomolar drug concentrations. Br J Clin Pharmacol 32: 167–172.
Di Ciano P, Underwood RJ, Hagan JJ, Everitt BJ (2003). Attenuation of cue-controlled cocaine-seeking by a selective D 3 dopamine receptor antagonist SB-277011-A. Neuropsychopharmacology 28: 329–338.
Dickerson BC, Eichenbaum H (2009). The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology 35: 86–104.
Eichenbaum H (2013). Memory on time. Trends Cogn Sci 17: 81–88.
Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489.
First MB (1997). User's guide for the structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. Amer Psychiatric Pub Inc: Washington, DC.
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I et al (2010). A selective role for dopamine in stimulus-reward learning. Nature 469: 53–57.
Floresco SB, Blaha CD, Yang CR, Phillips AG (2001). Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci 21: 2851–2860.
Floresco SB, Yang CR, Phillips AG, Blaha CD (1998). Association basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci 10: 1241–1251.
Frey U, Schroeder H, Matthies H (1990). Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res 522: 69–75.
Goto Y, Grace AA (2008). Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31: 552–558.
Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C et al (1996). Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93: 12040–12045.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997). Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. NeuroImage 6: 279–287.
Haber SN, Knutson B (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26.
Haber SN, McFarland NR (1999). The concept of the ventral striatum in nonhuman primates. Ann N Y Acad Sci 877: 33–48.
Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL (2003). Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berl) 168: 75–83.
Hitchcott PK, Phillips GD (1997). Amygdala and hippocampus control dissociable aspects of drug-associated conditioned rewards. Psychopharmacology 131: 187–195.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Ito R, Dalley JW, Robbins TW, Everitt BJ (2002). Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22: 6247–6253.
Jay TM (2003). Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Progr Neurobiol 69: 375–390.
Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, Epperson LE (1998). Reliability and validity of the cocaine selective severity assessment. Addict Behav 23: 449–461.
Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB (2002). Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci 22: 1126–1136.
Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F et al (2001). Neural activity related to drug craving in cocaine addiction. Archives General Psychiatry 58: 334.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. NeuroImage 4: 153–158.
Ledford CC, Fuchs RA, See RE (2003). Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology 28: 1721–1729.
Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C (2005). Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behav Neurosci 119: 1619–1627.
Li S, Cullen WK, Anwyl R, Rowan MJ (2003). Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci 6: 526–531.
Lingawi NW, Balleine BW (2012). Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J Neurosci 32: 1073–1081.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang D-R, Huang Y et al (2003). Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23: 285–300.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang D-R et al (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D2 receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057.
McBride D, Barrett SP, Kelly JT, Aw A, Dagher A (2006). Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31: 2728–2738.
McDonald RJ, White NM (1993). A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosc 107: 3–22.
Meil WM, See RE (1997). Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87: 139–148.
Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M et al (2002). Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 46: 170–188.
Nicola SM, Taha SA, Kim SW, Fields HL (2005). Nucleus accumbens dopamine release is necessary and sufficient to promote the behavioral response to reward-predictive cues. Neuroscience 135: 1025–1033.
Otmakhova NA, Lisman JE (1998). D1/D5 dopamine receptors inhibit depotentiation at CA1 synapses via cAMP-dependent mechanism. J Neurosci 18: 1270–1279.
O’Brien C, Greenstein R, Ternes J, McLellan A, Grabowski J (1979). Unreinforced self-injections: effects on rituals and outcome in heroin addicts. Probl Drug Depend 27: 275–281.
Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56: 907–922.
Phillips AG, Ahn S, Howland JG (2003). Amygdalar control of the mesocorticolimbic dopamine system: parallel pathways to motivated behavior. Neurosci Biobehav Rev 27: 543–554.
Prisciandaro JJ, McRae-Clark AL, Myrick H, Henderson S, Brady KT (2012). Brain activation to cocaine cues and motivation/treatment status. Addict Biol doi:10.1111/j.1369-1600.2012.00446.x.
Rayport M, Sani S, Ferguson SM (2006). Olfactory gustatory responses evoked by electrical stimulation of amygdalar region in man are qualitatively modifiable by interview content: case report and review. Int Rev Neurobiol 76: 35–42.
Robbins TW, Ersche KD, Everitt BJ (2008). Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 1141: 1–21.
Robbins TW, Everitt BJ (2002). Limbic-striatal memory systems and drug addiction. Neurobiol Learn Mem 78: 625–636.
Robinson TE, Flagel SB (2009). Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65: 869–873.
Rogers JL, See RE (2007). Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiol Learn Mem 87: 688–692.
Savage LM, Ramos RL (2009). Reward expectation alters learning and memory: The impact of the amygdala on appetitive-driven behaviors. Behav Brain Res 198: 1–12.
See RE, Kruzich PJ, Grimm JW (2001). Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 154: 301–310.
Shohamy D, Adcock RA (2010). Dopamine and adaptive memory. Trends Cogn Sci 14: 464–472.
Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J et al (2010). Striatal and extrastriatal dopamine release measured with PET and [18F] fallypride. Synapse 64: 350–362.
Staiger PK, White JM (1991). Cue reactivity in alcohol abusers: stimulus specificity and extinction of the responses. Addict Behav 16: 211–221.
Tang DW, Fellows LK, Small DM, Dagher A (2012). Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol Behav 106: 317–324.
Tracy AL, Jarrard LE, Davidson TL (2001). The hippocampus and motivation revisited: appetite and activity. Behav Brain Res 127: 13–23.
Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM et al (2012). Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 32: 6170–6176.
Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R (2009). Intact rapid detection of fearful faces in the absence of the amygdala. Nat Neurosci 12: 1224–1225.
Tye KM, Janak PH (2007). Amygdala neurons differentially encode motivation and reinforcement. J Neurosci 27: 3937–3945.
Vanderschuren LJMJ, Di Ciano P, Everitt BJ (2005). Involvement of the Dorsal Striatum in Cue-Controlled Cocaine Seeking. J Neurosci 25: 8665–8670.
Vernaleken I, Peters L, Raptis M, Lin R, Buchholz HG, Zhou Y et al (2011). The applicability of SRTM in [18F]fallypride PET investigations: impact of scan durations. J Cereb Blood Flow Metab 31: 1958–1966.
Volkow ND, Fowler JS, Wang G-J, Telang F, Logan J, Jayne M et al (2010). Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. NeuroImage 49: 2536–2543.
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR et al (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26: 6583.
Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL (2001). Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science 292: 1175–1178.
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O (2000). Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci 97: 4321.
Wertz JM, Sayette MA (2001). A review of the effects of perceived drug use opportunity on self-reported urge. Exp Clin Psychopharmacol 9: 3–13.
Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC (2001). Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 158: 86–95.
Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E (2005). Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron 45: 459–467.
Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A et al (2006). Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 31: 2716–2727.
Acknowledgements
We thank R Fukasawa, G Sauchuck, and S Mattei for technical assistance at the PET unit; D Jolly and M Kovacevic for preparation of radiotracers. Funding for the study came from an operating grant to ML from the Canadian Institutes of Health Research (CIHR, MOP-36429).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Fotros, A., Casey, K., Larcher, K. et al. Cocaine Cue-Induced Dopamine Release in Amygdala and Hippocampus: A High-Resolution PET [18F]Fallypride Study in Cocaine Dependent Participants. Neuropsychopharmacol 38, 1780–1788 (2013). https://doi.org/10.1038/npp.2013.77
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.77
Keywords
This article is cited by
-
Neural circuit selective for fast but not slow dopamine increases in drug reward
Nature Communications (2023)
-
The influence of conditioned stimuli on [11C]-(+)-PHNO PET binding in tobacco smokers after a one week abstinence
Scientific Reports (2021)
-
Multimodal investigation of dopamine D2/D3 receptors, default mode network suppression, and cognitive control in cocaine-use disorder
Neuropsychopharmacology (2021)
-
Memory enhancing effects of nicotine, cocaine, and their conditioned stimuli; effects of beta-adrenergic and dopamine D2 receptor antagonists
Psychopharmacology (2021)
-
Neurocircuitry of Mindfulness-Based Interventions for Substance Use Prevention and Recovery
Current Addiction Reports (2021)