Abstract
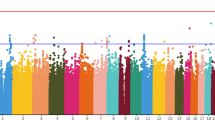
Methamphetamine (METH) use can provoke psychotic reactions requiring immediate treatment, namely METH-induced psychosis. Although the distinction between METH-induced and primary psychosis is important for understanding their clinical courses, we do not have clear diagnostic procedure by their symptoms. Not only are there similarities between the clinical features of METH-induced psychosis and schizophrenia (SCZ), but there is also epidemiological evidence of a shared genetic risk between ‘METH-related’ disorders and SCZ, which makes the differentiation of these two conditions difficult. In this study, we conducted a genome-wide association study (GWAS) targeting METH-dependent patients. The METH sample group, used in the METH-dependence GWAS, included 236 METH-dependent patients and 864 healthy controls. We also included a ‘within-case’ comparison between 194 METH-induced psychosis patients and 42 METH-dependent patients without psychosis in a METH-induced psychosis GWAS. To investigate the shared genetic components between METH dependence, METH-induced psychosis, and SCZ, data from our previous SCZ GWAS (total N=1108) were re-analyzed. In the SNP-based analysis, none of the SNPs showed genome-wide significance in either data set. By performing a polygenic component analysis, however, we found that a large number of ‘risk’ alleles for METH-induced psychosis are over-represented in individuals with SCZ (Pbest=0.0090). Conversely, we did not detect enrichment either between METH dependence and METH-induced psychosis or between METH dependence and SCZ. The results support previous epidemiological and neurobiological evidence for a relationship between METH-induced psychosis and SCZ. These also suggest that the overlap between genes scored as positive in these data sets can have higher probability as susceptibility genes for psychosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bousman CA, Glatt SJ, Everall IP, Tsuang MT (2009). Genetic association studies of methamphetamine use disorders: a systematic review and synthesis. Am J Med Genet B Neuropsychiatr Genet 150B: 1025–1049.
Callaghan RC, Cunningham JK, Allebeck P, Arenovich T, Sajeev G, Remington G et al (2012). Methamphetamine use and schizophrenia: a population-based cohort study in California. Am J Psychiatry 169: 389–396.
Chen CK, Lin SK, Sham PC, Ball D, Loh el W, Murray RM (2005). Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. Am J Med Genet B Neuropsychiatr Genet 136B: 87–91.
Cirulli ET, Goldstein DB (2010). Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 11: 415–425.
Clark SL, Adkins DE, Aberg K, Hettema JM, McClay JL, Souza RP et al (2012). Pharmacogenomic study of side-effects for antidepressant treatment options in STAR*D. Psychol Med 42: 1151–1162.
Grelotti DJ, Kanayama G, Pope HG Jr. (2010). Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: presentation of a case and review of the literature. Am J Psychiatry 167: 17–23.
Huang J, Perlis RH, Lee PH, Rush AJ, Fava M, Sachs GS et al (2010). Cross-disorder genomewide analysis of schizophrenia, bipolar disorder, and depression. Am J Psychiatry 167: 1254–1263.
Ikeda M, Aleksic B, Kinoshita Y, Okochi T, Kawashima K, Kushima I et al (2011). Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry 69: 472–478.
Ikeda M, Aleksic B, Yamada K, Iwayama-Shigeno Y, Matsuo K, Numata S et al (2012). Genetic evidence for association between NOTCH4 and schizophrenia supported by a GWAS follow-up study in a Japanese population. Mol Psychiatry (in press).
Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH (2006). Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry 11: 737–747 705.
Lavedan C, Licamele L, Volpi S, Hamilton J, Heaton C, Mack K et al (2009). Association of the NPAS3 gene and five other loci with response to the antipsychotic iloperidone identified in a whole genome association study. Mol Psychiatry 14: 804–819.
Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM et al (2010). A versatile gene-based test for genome-wide association studies. Am J Hum Genet 87: 139–145.
Machiyama Y (1992). Chronic methamphetamine intoxication model of schizophrenia in animals. Schizophr Bull 18: 107–113.
Macintyre G, Alford T, Xiong L, Rouleau GA, Tibbo PG, Cox DW (2010). Association of NPAS3 exonic variation with schizophrenia. Schizophr Res 120: 143–149.
MacLaren EJ, Charlesworth P, Coba MP, Grant SG (2011). Knockdown of mental disorder susceptibility genes disrupts neuronal network physiology in vitro. Mol Cell Neurosci 47: 93–99.
Miyagawa T, Kawashima M, Nishida N, Ohashi J, Kimura R, Fujimoto A et al (2008). Variant between CPT1B and CHKB associated with susceptibility to narcolepsy. Nat Genet 40: 1324–1328.
Nutt DJ, King LA, Phillips LD (2010). Drug harms in the UK: a multicriteria decision analysis. Lancet 376: 1558–1565.
Otowa T, Yoshida E, Sugaya N, Yasuda S, Nishimura Y, Inoue K et al (2009). Genome-wide association study of panic disorder in the Japanese population. J Hum Genet 54: 122–126.
Pickard BS, Christoforou A, Thomson PA, Fawkes A, Evans KL, Morris SW et al (2009). Interacting haplotypes at the NPAS3 locus alter risk of schizophrenia and bipolar disorder. Mol Psychiatry 14: 874–884.
Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, Wu LC et al (2005). The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci USA 102: 14052–14057.
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38: 904–909.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al (2007). PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–575.
Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF et al (2009). Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460: 748–752.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al (2009). Common variants conferring risk of schizophrenia. Nature 460: 744–747.
Sullivan PF, Lin D, Tzeng JY, van den Oord E, Perkins D, Stroup TS et al (2008). Genomewide association for schizophrenia in the CATIE study: results of stage 1. Mol Psychiatry 13: 570–584.
Uhl GR, Drgon T, Liu QR, Johnson C, Walther D, Komiyama T et al (2008). Genome-wide association for methamphetamine dependence: convergent results from 2 samples. Arch Gen Psychiatry 65: 345–355.
Ujike H, Sato M (2004). Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann N Y Acad Sci 1025: 279–287.
Yang J, Lee SH, Goddard ME, Visscher PM (2011). GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet 88: 76–82.
Acknowledgements
This work was supported by research grants from Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; Grants-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from MEXT of Japan; Ministry of Health, Labor and Welfare of Japan; Academic Frontier Project for Private Universities, Comparative Cognitive Science Institutes; Core Research for Evolutional Science and Technology; Uehara Memorial Foundation; SENSHIN Medical Research Foundation; Takeda Science Foundation; the Novartis Foundation, Japan; Naito Foundation, Japan; and Strategic Research Program for Brain Sciences of the MEXT of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Ikeda, M., Okahisa, Y., Aleksic, B. et al. Evidence for Shared Genetic Risk Between Methamphetamine-Induced Psychosis and Schizophrenia. Neuropsychopharmacol 38, 1864–1870 (2013). https://doi.org/10.1038/npp.2013.94
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.94
Keywords
This article is cited by
-
Methamphetamine (MA) use and MA-induced psychosis are associated with increasing aberrations in the compensatory immunoregulatory system, interleukin-1α, and CCL5 levels
Translational Psychiatry (2023)
-
Polygenic risk score: use in migraine research
The Journal of Headache and Pain (2018)
-
Novelty seeking mediates the effect of DRD3 variation on onset age of amphetamine dependence in Han Chinese population
European Archives of Psychiatry and Clinical Neuroscience (2018)
-
Comparison of demographic characteristics and psychiatric comorbidity among methamphetamine-, heroin- and methamphetamine-heroin co- dependent males in Hunan, China
BMC Psychiatry (2017)
-
Schizophrenia genetics in the genome-wide era: a review of Japanese studies
npj Schizophrenia (2017)