Abstract
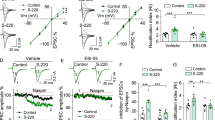
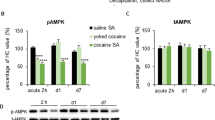
The mechanistic target of rapamycin complex 1 (mTORC1) is necessary for synaptic plasticity, as it is critically involved in the translation of synaptic transmission-related proteins, such as Ca2+/Calmodulin-dependent kinase II alpha (CAMKIIα) and AMPA receptor subunits (GluAs). Although recent studies have implicated mTORC1 signaling in drug-motivated behavior, the ineffectiveness of rapamycin, an mTORC1 inhibitor, in suppressing cocaine self-administration has raised questions regarding the specific role of mTORC1 in drug-related behaviors. Here, we examined mTORC1’s role in three drug-related behaviors: cocaine taking, withdrawal, and reinstatement of cocaine seeking, by measuring indices of mTORC1 activity and assessing the effect of intra-cerebroventricular rapamycin on these behaviors in rats. We found that withdrawal from cocaine self-administration increased indices of mTORC1 activity in the nucleus accumbens (NAC). Intra-cerebroventricular rapamycin attenuated progressive ratio (PR) break points and reduced phospho-p70 ribosomal S6 kinase, GluA1 AMPAR, and CAMKIIα levels in the NAC shell (NACsh) and core (NACc). In a subsequent study, we treated rats with intra-NACsh infusions of rapamycin (2.5 μg/side/day for 5 days) during cocaine self-administration and then tracked the expression of addiction-relevant behaviors through to withdrawal and extinction. Rapamycin reduced drug seeking in signaled non-drug-available periods, PR responding, and cue-induced reinstatement, with these effects linked to reduced mTORC1 activity, total CAMKIIα, and GluA1 AMPAR levels in the NACsh. Together, these data highlight a role for mTORC1 in the neural processes that control the expression and maintenance of drug reward, including protracted relapse vulnerability. These effects appear to involve a role for mTORC1 in the regulation of GluA1 AMPARs and CAMKIIα in the NACsh.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE et al (2008). CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 11: 344–353.
Bailey J, Ma D, Szumlinski KK (2012). Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol 17: 248–258.
Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V et al (2013). Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci 16: 1111–1117.
Blundell J, Kouser M, Powell CM (2008). Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem 90: 28–35.
Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J (2002). Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci 22: 8911–8921.
Brown AL, Flynn JR, Smith DW, Dayas CV (2011). Down-regulated striatal gene expression for synaptic plasticity-associated proteins in addiction and relapse vulnerable animals. Int J Neuropsychopharmacol 14: 1099–1110.
Cammalleri M, Lutjens R, Berton F, King AR, Simpson C, Francesconi W et al (2003). Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc Natl Acad Sci USA 100: 14368–14373.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Cota D, Matter EK, Woods SC, Seeley RJ (2008). The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28: 7202–7208.
Dayas CV, Smith DW, Dunkley PR (2012). An emerging role for the Mammalian target of rapamycin in ‘pathological’ protein translation: relevance to cocaine addiction. Front Pharmacol 3: 13.
Deroche-Gamonet V, Belin D, Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science 305: 1014–1017.
Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galinanes GL, Heng LJ et al (2011). Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 61: 1141–1151.
Hoeffer CA, Klann E (2010). mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75.
Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M et al (2013). mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 16: 441–448.
Ito R, Robbins TW, Everitt BJ (2004). Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci 7: 389–397.
James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV (2011a). Propensity to 'relapse' following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199: 235–242.
James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, Flynn JR et al (2010). Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One 5: e12980.
James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW et al (2011b). Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol 14: 684–690.
Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O et al (2010). Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328: 1709–1712.
Licata SC, Pierce RC (2003). The roles of calcium/calmodulin-dependent and Ras/mitogen-activated protein kinases in the development of psychostimulant-induced behavioral sensitization. J Neurochem 85: 14–22.
Lin J, Liu L, Wen Q, Zheng C, Gao Y, Peng S et al (2014). Rapamycin prevents drug seeking via disrupting reconsolidation of reward memory in rats. Int J Neuropsychopharmacol 17: 127–136.
Lisman J, Yasuda R, Raghavachari S (2012). Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci 13: 169–182.
Loweth JA, Baker LK, Guptaa T, Guillory AM, Vezina P (2008). Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci Lett 444: 157–160.
Mameli M, Balland B, Lujan R, Luscher C (2007). Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317: 530–533.
McGinty JF, Shi XD, Schwendt M, Saylor A, Toda S (2008). Regulation of psychostimulant-induced signaling and gene expression in the striatum. J Neurochem 104: 1440–1449.
Narita M, Akai H, Kita T, Nagumo Y, Narita M, Sunagawa N et al (2005). Involvement of mitogen-stimulated p70-S6 kinase in the development of sensitization to the methamphetamine-induced rewarding effect in rats. Neuroscience 132: 553–560.
Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D (2010). Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci USA 107: 20093–20098.
Opazo P, Labrecque S, Tigaret CM, Frouin A, Wiseman PW, De Koninck P et al (2010). CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67: 239–252.
Parsons RG, Gafford GM, Helmstetter FJ (2006). Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci 26: 12977–12983.
Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW (1998). Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J Pharmacol Exp Ther 286: 1171–1176.
Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ (2009). Neurotrophic factors and structural plasticity in addiction. Neuropharmacology 56 (Suppl 1)): 73–82.
Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME (2004). BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24: 7366–7377.
Shi J, Jun W, Zhao LY, Xue YX, Zhang XY, Kosten TR et al (2009). Effect of rapamycin on cue-induced drug craving in abstinent heroin addicts. Eur J Pharmacol 615: 108–112.
Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH (2009). BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One 4: e6007.
Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M (2011). Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci USA 108: 3791–3796.
Sun X, Milovanovic M, Zhao Y, Wolf ME (2008). Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28: 4216–4230.
Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J et al (2010a). Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology 35: 913–928.
Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF et al (2010b). Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30: 12632–12641.
Wolf ME (2010). The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci 33: 391–398.
Acknowledgements
We acknowledge the assistance of Mr Cameron Adams and Mr Jonathan Cvetanoski for their technical assistance with the behavioral component of the i.c.v. experiment. We also thank Mrs Helen Carpenter and Mrs Michelle McConachy for their technical assistance with the western-blot analyses. These studies were supported by funding from the Australian National Health and Medical Research Council, the Hunter Medical Research Institute, and the University of Newcastle through project grants to C.V.D.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
James, M., Quinn, R., Ong, L. et al. mTORC1 Inhibition in the Nucleus Accumbens ‘Protects’ Against the Expression of Drug Seeking and ‘Relapse’ and Is Associated with Reductions in GluA1 AMPAR and CAMKIIα Levels. Neuropsychopharmacol 39, 1694–1702 (2014). https://doi.org/10.1038/npp.2014.16
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.16
Keywords
This article is cited by
-
A functional eEF2K-eEF2 pathway in the NAc is critical for the expression of cocaine-induced psychomotor sensitisation and conditioned place preference
Translational Psychiatry (2022)
-
Ribosomal DNA transcription is increased in the left nucleus accumbens of heroin-dependent males
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
VTA mTOR Signaling Regulates Dopamine Dynamics, Cocaine-Induced Synaptic Alterations, and Reward
Neuropsychopharmacology (2018)
-
Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats
Translational Psychiatry (2015)