Abstract
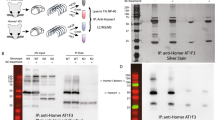
Stress-induced psychiatric disorders, such as depression, have recently been linked to changes in glutamate transmission in the central nervous system. Glutamate signaling is mediated by a range of receptors, including metabotropic glutamate receptors (mGluRs). In particular, mGluR subtype 5 (mGluR5) is highly implicated in stress-induced psychopathology. The major scaffold protein Homer1 critically interacts with mGluR5 and has also been linked to several psychopathologies. Yet, the specific role of Homer1 in this context remains poorly understood. We used chronic social defeat stress as an established animal model of depression and investigated changes in transcription of Homer1a and Homer1b/c isoforms and functional coupling of Homer1 to mGluR5. Next, we investigated the consequences of Homer1 deletion, overexpression of Homer1a, and chronic administration of the mGluR5 inverse agonist CTEP (2-chloro-4-((2,5-dimethyl-1-(4-(trifluoromethoxy)phenyl)-1H-imidazol-4-yl)ethynyl)pyridine) on the effects of chronic stress. In mice exposed to chronic stress, Homer1b/c, but not Homer1a, mRNA was upregulated and, accordingly, Homer1/mGluR5 coupling was disrupted. We found a marked hyperactivity behavior as well as a dysregulated hypothalamic–pituitary–adrenal axis activity in chronically stressed Homer1 knockout (KO) mice. Chronic administration of the selective and orally bioavailable mGluR5 inverse agonist, CTEP, was able to recover behavioral alterations induced by chronic stress, whereas overexpression of Homer1a in the hippocampus led to an increased vulnerability to chronic stress, reflected in an increased physiological response to stress as well as enhanced depression-like behavior. Overall, our results implicate the glutamatergic system in the emergence of stress-induced psychiatric disorders, and support the Homer1/mGluR5 complex as a target for the development of novel antidepressant agents.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J et al (2003). In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy-5-(pyridin-2-ylethynyl)pyridine). Eur J Pharmacol 473: 35–40.
Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF et al (2001). Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965.
Ary AW, Aguilar VR, Szumlinski KK, Kippin TE (2007). Prenatal stress alters limbo-corticostriatal Homer protein expression. Synapse 61: 938–941.
Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O et al (2013). Imbalances in prefrontal cortex CC-Homer1 versus CC-Homer2 expression promote cocaine preference. J Neurosci 33: 8101–8113.
Bertaso F, Roussignol G, Worley P, Bockaert J, Fagni L, Ango F (2010). Homer1a-dependent crosstalk between NMDA and metabotropic glutamate receptors in mouse neurons. PLoS One 5: e9755.
Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ et al (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–868.
Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL et al (1997). Homer: a protein that selectively binds metabotropic glutamate receptors. Nature 386: 284–288.
Busse CS, Brodkin J, Tattersall D, Anderson JJ, Warren N, Tehrani L et al (2004). The behavioral profile of the potent and selective mGlu5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine (MTEP) in rodent models of anxiety. Neuropsychopharmacology 29: 1971–1979.
Celikel T, Marx V, Freudenberg F, Zivkovic A, Resnik E, Hasan MT et al (2007). Select overexpression of homer1a in dorsal hippocampus impairs spatial working memory. Front Neurosci 1: 97–110.
Chrousos GP (2009). Stress and disorders of the stress system. Nat Rev Endocrinol 5: 374–381.
Connolly KR, Thase ME (2011). If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs 71: 43–64.
Cryan JF, Holmes A (2005). The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4: 775–790.
de Bartolomeis A, Iasevoli F (2003). The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull 37: 51–83.
de Kloet ER, Joëls M, Holsboer F (2005). Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475.
Grinevich V, Jezova D, Gambaryan S, Illarionova A, Kolleker A, Seeburg PH et al (2011). Hypertrophy and altered activity of the adrenal cortex in Homer 1 knockout mice. Horm Metab Res 43: 551–556.
Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M et al (2012). The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology 62: 332–339.
Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu RM et al (2009). The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell 137: 159–171.
Hong Q, Wang YP, Zhang M, Pan XQ, Guo M, Li F et al (2011). Homer expression in the hippocampus of an animal model of attention-deficit/hyperactivity disorder. Mol Med Rep 4: 705–712.
Hong Q, Zhang M, Pan XQ, Guo M, Li F, Tong ML et al (2009). Prefrontal cortex Homer expression in an animal model of attention-deficit/hyperactivity disorder. J Neurol Sci 287: 205–211.
Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF et al (2007). Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav 6: 141–154.
Joëls M, Baram TZ (2009). The neuro-symphony of stress. Nat Rev Neurosci 10: 459–466.
Kammermeier PJ (2008). Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci 28: 8560–8567.
Kammermeier PJ, Worley PF (2007). Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci USA 104: 6055–6060.
Kato A, Ozawa F, Saitoh Y, Hirai K, Inokuchi K (1997). vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett 412: 183–189.
Kavalali ET, Monteggia LM (2012). Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 169: 1150–1156.
Kendell SF, Krystal JH, Sanacora G (2005). GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets 9: 153–168.
Krystal JH, Mathew SJ, D'Souza DC, Garakani A, Gunduz-Bruce H, Charney DS (2010). Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs 24: 669–693.
Krystal JH, Sanacora G, Duman RS (2013). Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73: 1133–1141.
Lindemann L, Jaeschke G, Michalon A, Vieira E, Honer M, Spooren W et al (2011). CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther 339: 474–486.
Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH et al (2005). Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci 25: 11586–11594.
Mahan AL, Mou L, Shah N, Hu JH, Worley PF, Ressler KJ (2012). Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J Neurosci 32: 4651–4659.
Malkesman O, Scattoni ML, Paredes D, Tragon T, Pearson B, Shaltiel G et al (2010). The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol Psychiatry 67: 864–871.
Mathews DC, Henter ID, Zarate CA (2012). Targeting the glutamatergic system to treat major depressive disorder: rationale and progress to date. Drugs 72: 1313–1333.
McEwen BS (2004). Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci 1032: 1–7.
Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG et al (2012). Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74: 49–56.
Mutlu O, Gumuslu E, Ulak G, Celikyurt IK, Kokturk S, Kir HM et al (2012). Effects of fluoxetine, tianeptine and olanzapine on unpredictable chronic mild stress-induced depression-like behavior in mice. Life Sci 91: 1252–1262.
Nestler EJ, Hyman SE (2010). Animal models of neuropsychiatric disorders. Nat Neurosci 13: 1161–1169.
Palaniyappan L, Insole L, Ferrier N (2009). Combining antidepressants: a review of evidence. APT 15: 90–99.
Palucha A, Branski P, Szewczyk B, Wieronska J, Klak K, Pilc A (2005). Potential antidepressant-like effect of MTEP, a potent and highly selective mGluR5 antagonist. Pharmacol Biochem Behav 81: 901–906.
Pilc A, Chaki S, Nowak G, Witkin JM (2008). Mood disorders: regulation by metabotropic glutamate receptors. Biochem Pharmacol 75: 997–1006.
Popoli M, Yan Z, McEwen BS, Sanacora G (2012). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 13: 22–37.
Prins J, Olivier B, Korte SM (2011). Triple reuptake inhibitors for treating subtypes of major depressive disorder: the monoamine hypothesis revisited. Expert Opin Investig Drugs 20: 1107–1130.
Rietschel M, Mattheisen M, Frank J, Treutlein J, Degenhardt F, Breuer R et al (2010). Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol Psychiatry 68: 578–585.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D et al (2006). Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163: 1905–1917.
Sagvolden T, Russell VA, Aase H, Johansen EB, Farshbaf M (2005). Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry 57: 1239–1247.
Sanacora G, Treccani G, Popoli M (2012). Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62: 63–77.
Savignac HM, Finger BC, Pizzo RC, O'Leary OF, Dinan TG, Cryan JF (2011). Increased sensitivity to the effects of chronic social defeat stress in an innately anxious mouse strain. Neuroscience 192: 524–536.
Scharf SH, Sterlemann V, Liebl C, Müller MB, Schmidt MV (2013). Chronic social stress during adolescence: interplay of paroxetine treatment and ageing. Neuropharmacology 72C: 38–46.
Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D et al (2007). Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology 32: 417–429.
Schmidt MV, Trümbach D, Weber P, Wagner K, Scharf SH, Liebl C et al (2010). Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J Neurosci 30: 16949–16958.
Shiraishi-Yamaguchi Y, Furuichi T (2007). The Homer family proteins. Genome Biol 8: 206.
Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK et al (2005). Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav 4: 273–288.
Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M et al (2004). Homer proteins regulate sensitivity to cocaine. Neuron 43: 401–413.
Thase ME (2006). Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr 11: 12–21.
Tronson NC, Guzman YF, Guedea AL, Huh KH, Gao C, Schwarz MK et al (2010). Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear. Biol Psychiatry 68: 1007–1015.
Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P et al (1999). Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23: 583–592.
Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M et al (1998). Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 21: 717–726.
Wagner KV, Hartmann J, Mangold K, Wang XD, Labermaier C, Liebl C et al (2013). Homer1 mediates acute stress-induced cognitive deficits in the dorsal hippocampus. J Neurosci 33: 3857–3864.
Wagner KV, Marinescu D, Hartmann J, Wang XD, Labermaier C, Scharf SH et al (2012). Differences in FKBP51 regulation following chronic social defeat stress correlate with individual stress sensitivity: influence of paroxetine treatment. Neuropsychopharmacology 37: 2797–2808.
Wagner KV, Wang XD, Liebl C, Scharf SH, Müller MB, Schmidt MV (2011). Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology 36: 579–587.
Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH et al (2011). Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis 42: 300–310.
Webhofer C, Gormanns P, Reckow S, Lebar M, Maccarrone G, Ludwig T et al (2013). Proteomic and metabolomic profiling reveals time-dependent changes in hippocampal metabolism upon paroxetine treatment and biomarker candidates. J Psychiatr Res 47: 289–298.
Yim YS, Lee J, Kim GT, Song T, Kim CH, Kim DG (2012). Hippocampal mGluR5 predicts an occurrence of helplessness behavior after repetitive exposure to uncontrollable stress. Neurosci Lett 519: 62–66.
Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH et al (2003). Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114: 777–789.
Acknowledgements
We thank Kathrin Hafner for her excellent technical assistance. Homer1 KO mice were generously provided by Dr Paul Worley (Johns Hopkins University). Natalie Matosin thanks the Company of Biologists (UK) for their support in the form of a Traveling Fellowship. This study was supported by the Brain & Behavior Research Foundation NARSAD grant 17322. This study was also partially supported by the National Alliance for Research on Schizophrenia and Depression (grant no. 17322).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Wagner, K., Hartmann, J., Labermaier, C. et al. Homer1/mGluR5 Activity Moderates Vulnerability to Chronic Social Stress. Neuropsychopharmacol 40, 1222–1233 (2015). https://doi.org/10.1038/npp.2014.308
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.308
This article is cited by
-
Sleep-mediated regulation of reward circuits: implications in substance use disorders
Neuropsychopharmacology (2023)
-
Saikosaponin D exerts antidepressant effect by regulating Homer1-mGluR5 and mTOR signaling in a rat model of chronic unpredictable mild stress
Chinese Medicine (2022)
-
Phenotypes, mechanisms and therapeutics: insights from bipolar disorder GWAS findings
Molecular Psychiatry (2022)
-
Frontal cortex genetic ablation of metabotropic glutamate receptor subtype 3 (mGlu3) impairs postsynaptic plasticity and modulates affective behaviors
Neuropsychopharmacology (2021)
-
mGluR5-Mediated eCB Signaling in the Nucleus Accumbens Controls Vulnerability to Depressive-Like Behaviors and Pain After Chronic Social Defeat Stress
Molecular Neurobiology (2021)