Abstract
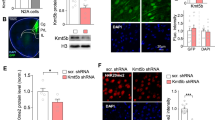
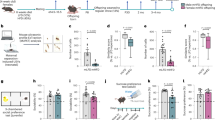
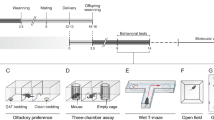
Poor-quality maternal diet during pregnancy, and subsequent gestational growth disturbances in the offspring, have been implicated in the etiology of multiple neurodevelopmental disorders, including ADHD, schizophrenia, and autism. These disorders are characterized, in part, by abnormalities in responses to reward and errors of executive function. Here, we demonstrate dissociable deficits in reward processing and executive function in male and female mice, solely due to maternal malnutrition via high-fat or low-protein diets. Gestational exposure to a high-fat diet delayed acquisition of a fixed ratio response, and decreased motivation as assessed by progressive ratio. In contrast, offspring of a low-protein diet displayed no deficits in operant learning, but were more prone to assign salience to a cue that predicts reward (sign-tracking) in a Pavlovian-conditioned approach task. In the 5-choice serial reaction time task (5-CSRTT), gestational exposure to a high-fat diet promoted impulsivity, whereas exposure to a low-protein diet led to marked inattention. These dissociable executive function deficits are known to be mediated by the medial prefrontal cortex (PFC), which displays markers of epigenetic dysregulation in neurodevelopmental disorders. Following behavioral characterization, we assayed PFC gene expression using a targeted PCR array and found that both maternal diets increased overall transcription in PFC. Cluster analysis of the relationships between individual transcripts and behavioral outcomes revealed a cluster of primarily epigenetic modulators, whose overexpression was linked to executive function deficits. The overexpression of four genes, DNA methyltransferase 1 (DNMT1), δ-opioid receptor (OPRD1), cannabinoid receptor 1 (CNR1), and catechol-o-methyltransferase (COMT), was strongly associated with overall poor performance. All 5-CSRTT deficits were associated with DNMT1 upregulation, whereas impulsive behavior could be dissociated from inattention by overexpression of OPRD1 or COMT, respectively, as well as a distinct cluster of epigenetic regulators. These data provide molecular support for dissociable domains of executive function.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arguello PA, Jentsch JD (2004). Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology (Berl) 177: 141–150.
Bari A, Robbins TW (2013). Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108: 44–79.
Ben Shalom D, Ronel Z, Faran Y, Meiri G, Gabis L, Kerns KA (2013). A double dissociation between inattentive and impulsive traits, on tasks of visual processing and emotion regulation. J Atten Disord e-pub ahead of print 10 December 2013 doi:10.1177/1087054713510351.
Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 1350: 43–64.
Black YD, Maclaren FR, Naydenov AV, Carlezon Wa, Baxter MG, Konradi C (2006). Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci 26: 9656–9665.
Bubnik MG, Hawk LW, Pelham WE, Waxmonsky JG, Rosch KS (2014). Reinforcement enhances vigilance among children with ADHD: comparisons to typically developing children and to the effects of methylphenidate. J Abnorm Child Psychol e-pub ahead of print 17 June 2014 doi:10.1007/s10802-014-9891-8.
Costas J, Sanjuán J, Ramos-Ríos R, Paz E, Agra S, Tolosa A et al (2011). Interaction between COMT haplotypes and cannabis in schizophrenia: a case-only study in two samples from Spain. Schizophr Res 127: 22–27.
Desplats P, Spencer B, Coffee E, Patel P, Michael S, Patrick C et al (2011). Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem 286: 9031–9037.
Duncan J (2013). The structure of cognition: attentional episodes in mind and brain. Neuron 80: 35–50.
Eisen MB, Spellman PT, Brown PO, Botstein D (1998). Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95: 14863–14868.
Epstein JN, Erkanli A, Conners CK, Klaric J, Costello JE, Angold A (2003). Relations between continuous performance test performance measures and ADHD behaviors. J Abnorm Child Psychol 31: 543–554.
Floresco SB, Jentsch JD (2011). Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology 36: 227–250.
Gray JA, Rickwood L, Drewett RF, Dunne E (1977). Gonadal hormones and effects of partial reinforcement on appetitive behaviour in the rat. Physiol Behav 19: 41–45.
Grayson DR, Guidotti A (2013). The dynamics of DNA methylation in schizophrenia and related psychiatric disorders. Neuropsychopharmacology 38: 138–166.
Grissom NM, Lyde R, Christ L, Sasson IE, Carlin J, Vitins AP et al (2013). Obesity at conception programs the opioid system in the offspring brain. Neuropsychopharmacology 39: 801–810.
Grissom NM, Reyes TM (2012). Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics. Int J Dev Neurosci 31: 406–414.
Hest A Van, Haaren F Van (1989). The effects of gonadectomy and chronic testosterone suppletion on the autoshaped response of male and female Wistar rats. Bull Psychon Soc 27: 9–12.
Johnson KA, Robertson IH, Kelly SP, Silk TJ, Barry E, Dáibhis A et al (2007). Dissociation in performance of children with ADHD and high-functioning autism on a task of sustained attention. Neuropsychologia 45: 2234–2245.
Klenowski P, Morgan M, Bartlett SE (2014). The role of delta opioid receptors in learning and memory underlying the development of addiction. Br J Pharmacol e-pub ahead of print 12 February 2014 doi:10.1111/bph.12618.
Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL et al (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics 129: e1121–e1128.
Kritzer MF, Brewer A, Montalmant F, Davenport M, Robinson JK (2007). Effects of gonadectomy on performance in operant tasks measuring prefrontal cortical function in adult male rats. Horm Behav 51: 183–194.
Kuntsi J, Pinto R, Price TS, van der Meere JJ, Frazier-Wood AC, Asherson P (2013). The separation of ADHD inattention and hyperactivity-impulsivity symptoms: pathways from genetic effects to cognitive impairments and symptoms. J Abnorm Child Psychol 42: 127–136.
Ladd-Acosta C, Hansen KD, Briem E, Fallin MD, Kaufmann WE, Feinberg AP (2013). Common DNA methylation alterations in multiple brain regions in autism. Mol Psychiatry 19: 862–871.
Mastroeni D, Chouliaras L, Grover A, Liang WS, Hauns K, Rogers J et al (2013). Reduced RAN expression and disrupted transport between cytoplasm and nucleus; a key event in Alzheimer’s disease pathophysiology. PLoS One 8: e53349.
McLaughlin RJ, Hill MN, Gorzalka BB (2014). A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci Biobehav Rev 42C: 116–131.
McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG (2013). The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One 8: e62189.
Moore GS, Kneitel AW, Walker CK, Gilbert WM, Xing G (2012). Autism risk in small- and large-for-gestational-age infants. Am J Obstet Gynecol 206: 314.e1–9.
Olmstead MC, Ouagazzal A-M, Kieffer BL (2009). Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One 4: e4410.
Ornoy A (2011). Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 32: 205–212.
Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M (2013). Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci 33: 8321–8335.
Pennanen L, van der Hart M, Yu L, Tecott LH (2013). Impact of serotonin (5-HT)2C receptors on executive control processes. Neuropsychopharmacology 38: 957–967.
Pezze M, McGarrity S, Mason R, Fone KC, Bast T (2014). Too little and too much: hypoactivation and disinhibition of medial prefrontal cortex cause attentional deficits. J Neurosci 34: 7931–7946.
Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF (1998). Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22: 1564–1569.
Roffman JL, Weiss AP, Deckersbach T, Freudenreich O, Henderson DC, Wong DH et al (2008). Interactive effects of COMT Val108/158Met and MTHFR C677T on executive function in schizophrenia. Am J Med Genet B Neuropsychiatr Genet 147B: 990–995.
Saunders BT, Robinson TE (2012). The role of dopamine in the accumbens core in the expression of pavlovian-conditioned responses. Eur J Neurosci 36: 2521–2532.
Saunders BT, Robinson TE (2013). Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav Rev 37: 1955–1975.
Schmittgen TD, Livak KJ (2008). Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3: 1101–1108.
Scott D, Taylor JR (2014). Chronic nicotine attenuates phencyclidine-induced impulsivity in a mouse serial reaction time task. Behav Brain Res 259: 164–173.
Slusarek M, Velling S, Bunk D, Eggers C (2001). Motivational effects on inhibitory control in children with ADHD. J Am Acad Child Adolesc Psychiatry 40: 355–363.
Tomie A, Lincks M, Nadarajah SD, Pohorecky La, Yu L (2011). Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behav Brain Res 226: 1–8.
Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD (2005). Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci USA 102: 12212–12217.
Van Lieshout R, Boyle M (2011). Canadian youth born large or small for gestational age and externalizing and internalizing problems. Can J Psychiatry 56: 227–234.
Van Lieshout RJ, Taylor VH, Boyle MH (2011). Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes Rev 12: e548–e559.
Vesco KK, Sharma AJ, Dietz PM, Rizzo JH, Callaghan WM, England L et al (2011). Newborn size among obese women with weight gain outside the 2009 Institute of Medicine recommendation. Obstet Gynecol 117: 812–818.
Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, Reyes TM (2010a). Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 151: 1–9.
Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I et al (2010b). Early life protein restriction alters dopamine circuitry. Neuroscience 168: 359–370.
Whitaker KW, Totoki K, Reyes TM (2011). Metabolic adaptations to early life protein restriction differ by offspring sex and post-weaning diet in the mouse. Nutr Metab Cardiovasc Dis 22: 1067–1074.
Young JW, Light GA, Marston HM, Sharp R, Geyer MA (2009). The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One 4: e4227.
Young JW, Meves JM, Tarantino IS, Caldwell S, Geyer MA (2011). Delayed procedural learning in α7-nicotinic acetylcholine receptor knockout mice. Genes Brain Behav 10: 720–733.
Acknowledgements
We acknowledge the technical support of Ariel Miller, Sarah McKee, Robert George, and Emily Shuldiner, and the helpful comments of Dr Lucia Peixoto, Dr Benjamin Saunders, and Dr Jared Young in preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Grissom, N., Herdt, C., Desilets, J. et al. Dissociable Deficits of Executive Function Caused by Gestational Adversity are Linked to Specific Transcriptional Changes in the Prefrontal Cortex. Neuropsychopharmacol 40, 1353–1363 (2015). https://doi.org/10.1038/npp.2014.313
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.313
This article is cited by
-
Prenatal environmental exposures associated with sex differences in childhood obesity and neurodevelopment
BMC Medicine (2023)
-
Age-dependent effects of protein restriction on dopamine release
Neuropsychopharmacology (2021)
-
Microglial and peripheral immune priming is partially sexually dimorphic in adolescent mouse offspring exposed to maternal high-fat diet
Journal of Neuroinflammation (2020)
-
Let’s call the whole thing off: evaluating gender and sex differences in executive function
Neuropsychopharmacology (2019)
-
Male-specific deficits in natural reward learning in a mouse model of neurodevelopmental disorders
Molecular Psychiatry (2018)