Abstract
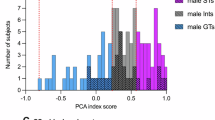
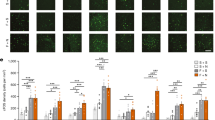
A discrete cue associated with intravenous injections of cocaine acquires greater control over motivated behavior in some rats (‘sign-trackers’, STs) than others (‘goal-trackers’, GTs). It is not known, however, if such variation generalizes to cues associated with other drugs. We asked, therefore, whether a discrete cue (a light) associated with the intravenous administration of an opioid drug (the short-acting mu receptor agonist, remifentanil) acquires incentive motivational properties differently in STs and GTs, as indicated by tests of Pavlovian conditioned approach and conditioned reinforcement. Consistent with studies using cocaine, STs approached a classically conditioned opioid cue more readily than GTs, and in a test of conditioned reinforcement worked more avidly to get it. Interestingly, STs and GTs did not differ in the acquisition of a conditioned orienting response. In addition, the performance of conditioned approach behavior, but not conditioned orientation, was attenuated by pretreatment with the dopamine receptor antagonist, flupenthixol, into the core of the nucleus accumbens. Lastly, food and opioid cues engaged similar amygdalo–striatal–thalamic circuitry to a much greater extent in STs than GTs, as indicated by Fos expression. Taken together, these data demonstrate that, similar to food and cocaine cues: (1) a discrete opioid cue attains greater incentive motivational value in STs than GTs; (2) the attribution of incentive motivational properties to an opioid cue is dopamine dependent; and (3) an opioid cue engages the so-called ‘motive circuit’ only if it is imbued with incentive salience.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Badiani A, Belin D, Epstein D, Calu D, Shaham Y (2011). Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci 12: 685–700.
Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ (2006). Individual differences in reward drive predict neural responses to images of food. J Neurosci 26: 5160–5166.
Bertz JW, Chen J, Woods JH (2014). Effects of pramipexole on the acquisition of responding with opioid-conditioned reinforcement in the rat. Psychopharmacology (Berl) e-pub ahead of print 3 July 2014 doi:10.1007/s00213-014-3659-2.
Bertz JW, Woods JH (2013). Acquisition of responding with a remifentanil-associated conditioned reinforcer in the rat. Psychopharmacology (Berl) 229: 235–243.
Boakes R (1977). Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwits H, (eds) Operant-Pavlovian Interactions. Lawrence Erlbaum Associates: Hillsdale. pp 67–97.
Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y (2007). Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27: 12655–12663.
Chang SE, Wheeler DS, Holland PC (20122012). Effects of lesions of the amygdala central nucleus on autoshaped lever pressing. Brain Res 1450: 49–56.
Chang SE, Wheeler DS, Holland PC (20122012). Roles of nucleus accumbens and basolateral amygdala in autoshaped lever pressing. Neurobiol Learn Mem 97: 441–451.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Danna CL, Shepard PD, Elmer GI (2013). The habenula governs the attribution of incentive salience to reward predictive cues. Front Hum Neurosci 7: 781.
Davis WM, Smith SG (1976). Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlovian J Biol Sci 11: 222–236.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
DiFeliceantonio AG, Berridge KC (2012). Which cue to 'want'? Opioid stimulation of central amygdala makes goal-trackers show stronger goal-tracking, just as sign-trackers show stronger sign-tracking. Behav Brain Res 230: 399–408.
Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982). Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 78: 204–209.
Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V et al (2013). Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS ONE 8: e75042.
Flagel SB, Cameron CM, Pickup KN, Watson SJ, Akil H, Robinson TE (20112011). A food predictive cue must be attributed with incentive salience for it to induce c-fos mRNA expression in cortico-striatal-thalamic brain regions. Neuroscience 196: 80–96.
Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I et al (20112011). A selective role for dopamine in stimulus-reward learning. Nature 469: 53–57.
Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P et al (2010). An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: Implications for addiction. Neuropsychopharmacology 35: 388–400.
Flagel SB, Watson SJ, Robinson TE, Akil H (2007). Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 191: 599–607.
Frohmader KS, Wiskerke J, Wise RA, Lehman MN, Coolen LM (2010). Methamphetamine acts on subpopulations of neurons regulating sexual behavior in male rats. Neuroscience 166: 771–784.
Gerrits MA, Ramsey NF, Wolterink G, van Ree JM (1994). Lack of evidence for an involvement of nucleus accumbens dopamine D1 receptors in the initiation of heroin self-administration in the rat. Psychopharmacology (Berl) 114: 486–494.
Haidar SH, Moreton JE, Liang Z, Hoke JF, Muir KT, Eddington ND (1997). Evaluating a possible pharmacokinetic interaction between remifentanil and esmolol in the rat. J Pharm Sci 86: 1278–1282.
Haight JL, Flagel SB (2014). A potential role for the paraventricular nucleus of the thalamus in mediating individual variation in Pavlovian conditioned responses. Front Behav Neurosci 8: 79.
Hearst E, Jenkins HM (1974) Sign Tracking: the Stimulus-Reinforcer Relation and Directed Action. Monograph of the Psychonomic Society: Austin.
Janes AC, Pizzagalli DA, Richardt S, Frederick Bde B, Holmes AJ, Sousa J et al (2010). Neural substrates of attentional bias for smoking-related cues: an FMRI study. Neuropsychopharmacology 35: 2339–2345.
Kelley AE, Schiltz CA, Landry CF (2005). Neural systems recruited by drug- and food-related cues: Studies of gene activation in corticolimbic regions. Physiol Behav 86: 11–14.
Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM et al (2014). Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology 39: 1135–1147.
Lai M, Chen W, Zhu H, Zhou X, Liu H, Zhang F et al (2013). Low dose risperidone attenuates cue-induced but not heroin-induced reinstatement of heroin seeking in an animal model of relapse. Int J Neuropsychopharmacol 16: 1569–1575.
Lyness WH, Friedle NM, Moore KE (1979). Destruction of dopaminergic nerve terminals in nucleus accumbens: effect on d-amphetamine self-administration. Pharmacol Biochem Behav 11: 553–556.
Madsen HB, Ahmed SH (2014). Drug versus sweet reward: greater attraction to and preference for sweet versus drug cues. Addict Biol e-pub ahead of print 7 March 2014 doi:10.1111/adb.12134.
Madsen HB, Brown RM, Short JL, Lawrence AJ (2012). Investigation of the neuroanatomical substrates of reward seeking following protracted abstinence in mice. J Physiol 590: 2427–2442.
Mahler SV, Berridge KC (2009). Which cue to ‘want?’ Central amygdala opioid activation enhances and focuses incentive salience on a prepotent reward cue. J Neurosci 29: 6500–6513.
Mahler SV, de Wit H (2010). Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PLoS ONE 5: e15475.
Maldonado R, Robledo P, Chover AJ, Caine SB, Koob GF (1993). D1 dopamine receptors in the nucleus accumbens modulate cocaine self-administration in the rat. Pharmacol Biochem Behav 45: 239–242.
Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD et al (2012). Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS ONE 7: e38987.
Peters J, De Vries TJ (2013). Pavlovian conditioned approach, extinction, and spontaneous recovery to an audiovisual cue paired with an intravenous heroin infusion. Psychopharmacology (Berl) 231: 447–453.
Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984). Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology (Berl) 84: 167–173.
Roberts DC, Koob GF, Klonoff P, Fibiger HC (1980). Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav 12: 781–787.
Robinson TE, Flagel SB (2009). Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry 65: 869–873.
Robinson TE, Yager LM, Cogan ES, Saunders BT (2014). On the motivational properties of reward cues: individual differences. Neuropharmacology 76: 450–459.
Saunders BT, Robinson TE (2010). A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry 67: 730–736.
Saunders BT, Robinson TE (2012). The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci 36: 2521–2532.
Saunders BT, Robinson TE (20132013). Individual variation in resisting temptation: implications for addiction. Neurosci Biobehav R 37: 1955–1975.
Saunders BT, Yager LM, Robinson TE (20132013). Cue-evoked cocaine ‘craving’: role of dopamine in the accumbens core. J Neurosci 33: 13989–14000.
Shalev U, Grimm JW, Shaham Y (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1–42.
Tang DW, Fellows LK, Small DM, Dagher A (2012). Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav 106: 317–324.
Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND (2014). Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum Brain Mapp e-pub ahead of print 21 August 2014 doi:10.1002/hbm.22617.
Wise RA, Bozarth MA (1987). A psychomotor stimulant theory of addiction. Psychol Rev 94: 469–492.
Yager LM, Robinson TE (2010). Cue-induced reinstatement of food seeking in rats that differ in their propensity to attribute incentive salience to food cues. Behav Brain Res 214: 30–34.
Yager LM, Robinson TE (2013). A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 226: 217–228.
Acknowledgements
We thank Anusari Dewasurendra, Kyle Eaton, and Elizabeth O’Donnell for assistance with behavioral testing.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse of the National Institutes of Health.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Yager, L., Pitchers, K., Flagel, S. et al. Individual Variation in the Motivational and Neurobiological Effects of an Opioid Cue. Neuropsychopharmacol 40, 1269–1277 (2015). https://doi.org/10.1038/npp.2014.314
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.314
This article is cited by
-
The propensity to sign-track is associated with externalizing behavior and distinct patterns of reward-related brain activation in youth
Scientific Reports (2023)
-
Nicotinic and muscarinic acetylcholine receptor antagonism dose-dependently decreases sign- but not goal-tracking behavior in male rats
Psychopharmacology (2023)
-
Reinstatement of Pavlovian responses to alcohol cues by stress
Psychopharmacology (2023)
-
Investigating discriminative stimulus modulation of opioid seeking after conflict-induced abstinence in sign- and goal-tracking rats
Psychopharmacology (2022)
-
Investigating individual differences in opioid-taking and opioid-seeking behavior in male rats
Psychopharmacology (2022)