Abstract
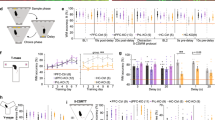
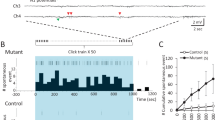
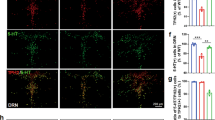
Both the glutamatergic and serotonergic (5-HT) systems are implicated in the modulation of mood and anxiety. Descending cortical glutamatergic neurons regulate 5-HT neuronal activity in the midbrain raphe nuclei through α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors. To analyze the functional role of GLUA1-containing AMPA receptors in serotonergic neurons, we used the Cre-ERT2/loxP-system for the conditional inactivation of the GLUA1-encoding Gria1 gene selectively in 5-HT neurons of adult mice. These Gria15-HT−/− mice exhibited a distinct anxiety phenotype but showed no alterations in locomotion, depression-like behavior, or learning and memory. Increased anxiety-related behavior was associated with significant decreases in tryptophan hydroxylase 2 (TPH2) expression and activity, and subsequent reductions in tissue levels of 5-HT, its metabolite 5-hydroxyindoleacetic acid (5-HIAA), and norepinephrine in the raphe nuclei. However, TPH2 expression and activity as well as monoamine levels were unchanged in the projection areas of 5-HT neurons. Extracellular electrophysiological recordings of 5-HT neurons revealed that, while α1-adrenoceptor-mediated excitation was unchanged, excitatory responses to AMPA were enhanced and the 5-HT1A autoreceptor-mediated inhibitory response to 5-HT was attenuated in Gria15-HT−/− mice. Our data show that a loss of GLUA1 protein in 5-HT neurons enhances AMPA receptor function and leads to multiple local molecular and neurochemical changes in the raphe nuclei that dysregulate 5-HT neuronal activity and induce anxiety-like behavior.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aghajanian GK, Vandermaelen CP, Andrade R (1983). Intracellular studies on the role of calcium in regulating the activity and reactivity of locus coeruleus neurons in vivo. Brain Res 273: 237–243.
Aguado C, Fernandez-Alacid L, Cabanero MJ, Yanagawa Y, Schilling K, Watanabe M et al (2013). Differential maturation of GIRK2-expressing neurons in the mouse cerebellum. J Chem Neuroanat 47: 79–89.
Ayissi Mbomo R, Gartside S, Ngo Bum E, Njikam N, Okello E, McQuade R (2012). Effect of Mimosa pudica (Linn.) extract on anxiety behaviour and GABAergic regulation of 5-HT neuronal activity in the mouse. J Psychopharmacol 26: 575–583.
Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G et al (2003). Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci 23: 6304–6314.
Bannerman DM, Deacon RM, Brady S, Bruce A, Sprengel R, Seeburg PH et al (2004). A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behav Neurosci 118: 643–647.
Baraban JM, Aghajanian GK (1980). Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology 19: 355–363.
Barkus C, Feyder M, Graybeal C, Wright T, Wiedholz L, Izquierdo A et al (2012). Do GluA1 knockout mice exhibit behavioral abnormalities relevant to the negative or cognitive symptoms of schizophrenia and schizoaffective disorder? Neuropharmacology 62: 1263–1272.
Berger SM, Weber T, Perreau-Lenz S, Vogt MA, Gartside SE, Maser-Gluth C et al (2012). A functional Tph2 C1473G polymorphism causes an anxiety phenotype via compensatory changes in the serotonergic system. Neuropsychopharmacology 37: 1986–1998.
Browne CA, Clarke G, Dinan TG, Cryan JF (2011). Differential stress-induced alterations in tryptophan hydroxylase activity and serotonin turnover in two inbred mouse strains. Neuropharmacology 60: 683–691.
Carkaci-Salli N, Salli U, Kuntz-Melcavage KL, Pennock MM, Ozgen H, Tekin I et al (2011). TPH2 in the ventral tegmental area of the male rat brain. Brain Res Bull 84: 376–380.
Carver CS, Miller CJ (2006). Relations of serotonin function to personality: current views and a key methodological issue. Psychiatry Res 144: 1–15.
Celada P, Puig MV, Martin-Ruiz R, Casanovas JM, Artigas F (2002). Control of the serotonergic system by the medial prefrontal cortex: potential role in the etiology of PTSD and depressive disorders. Neurotox Res 4: 409–419.
Chourbaji S, Brandwein C, Vogt MA, Dormann C, Gass P (2008a). Evaluation of effects of previous exposure to an acute stressor before testing for depression-like behaviours in mice. Stress 11: 170–175.
Chourbaji S, Brandwein C, Vogt MA, Dormann C, Hellweg R, Gass P (2008b). Nature vs. nurture: can enrichment rescue the behavioural phenotype of BDNF heterozygous mice? Behav Brain Res 192: 254–258.
Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M et al (2006). IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis 23: 587–594.
Chourbaji S, Vogt MA, Fumagalli F, Sohr R, Frasca A, Brandwein C et al (2008c). AMPA receptor subunit 1 (GluR-A) knockout mice model the glutamate hypothesis of depression. FASEB J 22: 3129–3134.
Chourbaji S, Zacher C, Sanchis-Segura C, Dormann C, Vollmayr B, Gass P (2005). Learned helplessness: validity and reliability of depressive-like states in mice. Brain Res Brain Res Protoc 16: 70–78.
Cools R, Roberts AC, Robbins TW (2008). Serotoninergic regulation of emotional and behavioural control processes. Trends Cogn Sci 12: 31–40.
Dayan P, Huys QJ (2009). Serotonin in affective control. Annu Rev Neurosci 32: 95–126.
Dingledine R, Borges K, Bowie D, Traynelis SF (1999). The glutamate receptor ion channels. Pharmacol Rev 51: 7–61.
Donaldson ZR, Piel DA, Santos TL, Richardson-Jones J, Leonardo ED, Beck SG et al (2013). Developmental effects of serotonin 1A autoreceptors on anxiety and social behavior. Neuropsychopharmacology 39: 291–302.
Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B et al (2008). Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron 59: 497–508.
Fairchild G, Leitch MM, Ingram CD (2003). Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology 45: 925–934.
Finger EC, Marsh AA, Buzas B, Kamel N, Rhodes R, Vythilingham M et al (2007). The impact of tryptophan depletion and 5-HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks. Neuropsychopharmacology 32: 206–215.
Fitzgerald PJ, Barkus C, Feyder M, Wiedholz LM, Chen YC, Karlsson RM et al (2010). Does gene deletion of AMPA GluA1 phenocopy features of schizoaffective disorder? Neurobiol Dis 40: 608–621.
Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA et al (1993). WAY100135: a novel, selective antagonist at presynaptic and postsynaptic 5-HT1A receptors. Eur J Pharmacol 237: 283–291.
Fuss J, Ben Abdallah NM, Hensley FW, Weber KJ, Hellweg R, Gass P (2010). Deletion of running-induced hippocampal neurogenesis by irradiation prevents development of an anxious phenotype in mice. Plos one 5:e12769.
Gartside SE, Cole AJ, Williams AP, McQuade R, Judge SJ (2007). AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei. Eur J Neurosci 25: 3001–3008.
Geyer MA, Vollenweider FX (2008). Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci 29: 445–453.
Gold SJ, Ambros-Ingerson J, Horowitz JR, Lynch G, Gall CM (1997). Stoichiometries of AMPA receptor subunit mRNAs in rat brain fall into discrete categories. J Comp Neurol 385: 491–502.
Goodwin GM, De Souza RJ, Green AR (1985a). The pharmacology of the hypothermic response in mice to 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT). A model of presynaptic 5-HT1 function. Neuropharmacology 24: 1187–1194.
Goodwin GM, De Souza RJ, Green AR (1985b). Presynaptic serotonin receptor-mediated response in mice attenuated by antidepressant drugs and electroconvulsive shock. Nature 317: 531–533.
Gordon JA, Hen R (2004). Genetic approaches to the study of anxiety. Annu Rev Neurosci 27: 193–222.
Gutknecht L, Araragi N, Merker S, Waider J, Sommerlandt FM, Mlinar B et al (2012). Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS ONE 7: e43157.
Gutknecht L, Kriegebaum C, Waider J, Schmitt A, Lesch KP (2009). Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain: convergent data from Tph2 knockout mice. Eur Neuropsychopharmacol 19: 266–282.
Hellweg R, Zueger M, Fink K, Hortnagl H, Gass P (2007). Olfactory bulbectomy in mice leads to increased BDNF levels and decreased serotonin turnover in depression-related brain areas. Neurobiol Dis 25: 1–7.
Heninger GR (1997). Serotonin, sex, and psychiatric illness. Proc Natl Acad Sci USA 94: 4823–4824.
Inta D, Vogt MA, Elkin H, Weber T, Lima-Ojeda JM, Schneider M et al (2013). Phenotype of mice with inducible ablation of GluA1 AMPA receptors during late adolescence: relevance for mental disorders. Hippocampus 24: 424–435.
Jacobs BL, Azmitia EC (1992). Structure and function of the brain serotonin system. Physiol Rev 72: 165–229.
Jensen V, Kaiser KM, Borchardt T, Adelmann G, Rozov A, Burnashev N et al (2003). A juvenile form of postsynaptic hippocampal long-term potentiation in mice deficient for the AMPA receptor subunit GluR-A. J Physiol 553: 843–856.
Johnson DA, Gartside SE, Ingram CD (2002). 5-HT1A receptor-mediated autoinhibition does not function at physiological firing rates: evidence from in vitro electrophysiological studies in the rat dorsal raphe nucleus. Neuropharmacology 43: 959–965.
Kelai S, Renoir T, Chouchana L, Saurini F, Hanoun N, Hamon M et al (2008). Chronic voluntary ethanol intake hypersensitizes 5-HT(1A) autoreceptors in C57BL/6J mice. J Neurochem 107: 1660–1670.
Lau T, Schneidt T, Heimann F, Gundelfinger ED, Schloss P (2010). Somatodendritic serotonin release and re-uptake in mouse embryonic stem cell-derived serotonergic neurons. Neurochem Int 57: 969–978.
Leonardo ED, Hen R (2008). Anxiety as a developmental disorder. Neuropsychopharmacology 33: 134–140.
Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D et al (2003). Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci 22: 430–440.
Levine ES, Jacobs BL (1992). Neurochemical afferents controlling the activity of serotonergic neurons in the dorsal raphe nucleus: microiontophoretic studies in the awake cat. J Neurosci 12: 4037–4044.
Lucki I (1998). The spectrum of behaviors influenced by serotonin. Biol Psychiatry 44: 151–162.
Mann JJ (2003). Neurobiology of suicidal behaviour. Nat Rev Neurosci 4: 819–828.
McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R (2001). The use of behavioral test batteries: effects of training history. Physiol Behav 73: 705–717.
Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M (2006). Inducible gene deletion in astroglia and radial glia—a valuable tool for functional and lineage analysis. Glia 54: 21–34.
Pardo CA, Eberhart CG (2007). The neurobiology of autism. Brain Pathol 17: 434–447.
Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A et al (2011). Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci 31: 6008–6018.
Rogers RD (2011). The roles of dopamine and serotonin in decision making: evidence from pharmacological experiments in humans. Neuropsychopharmacology 36: 114–132.
Sakai K, Crochet S (2000). Serotonergic dorsal raphe neurons cease firing by disfacilitation during paradoxical sleep. Neuroreport 11: 3237–3241.
Sanderson DJ, Bannerman DM (2012). The role of habituation in hippocampus-dependent spatial working memory tasks: evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus 22: 981–994.
Sanderson DJ, Good MA, Seeburg PH, Sprengel R, Rawlins JN, Bannerman DM (2008). The role of the GluR-A (GluR1) AMPA receptor subunit in learning and memory. Prog Brain Res 169: 159–178.
Sanderson DJ, Hindley E, Smeaton E, Denny N, Taylor A, Barkus C et al (2011). Deletion of the GluA1 AMPA receptor subunit impairs recency-dependent object recognition memory. Learn Mem 18: 181–190.
Sato K, Kiyama H, Tohyama M (1993). The differential expression patterns of messenger RNAs encoding non-N-methyl-D-aspartate glutamate receptor subunits (GluR1-4) in the rat brain. Neuroscience 52: 515–539.
Strekalova T, Zorner B, Zacher C, Sadovska G, Herdegen T, Gass P (2003). Memory retrieval after contextual fear conditioning induces c-Fos and JunB expression in CA1 hippocampus. Genes Brain Behav 2: 3–10.
Tao R, Auerbach SB (2000). Regulation of serotonin release by GABA and excitatory amino acids. J Psychopharmacol 14: 100–113.
Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J et al (2007). A molecular basis of analgesic tolerance to cannabinoids. J Neurosci 27: 4165–4177.
Vekovischeva OY, Aitta-Aho T, Echenko O, Kankaanpaa A, Seppala T, Honkanen A et al (2004). Reduced aggression in AMPA-type glutamate receptor GluR-A subunit-deficient mice. Genes Brain Behav 3: 253–265.
Vekovischeva OY, Zamanillo D, Echenko O, Seppala T, Uusi-Oukari M, Honkanen A et al (2001). Morphine-induced dependence and sensitization are altered in mice deficient in AMPA-type glutamate receptor-A subunits. J Neurosci 21: 4451–4459.
Vogt MA, Chourbaji S, Brandwein C, Dormann C, Sprengel R, Gass P (2008). Suitability of tamoxifen-induced mutagenesis for behavioral phenotyping. Exp Neurol 211: 25–33.
Vogt MA, Elkin H, Pfeiffer N, Sprengel R, Gass P, Inta D (2014). Impact of adolescent GluA1 AMPA receptor ablation in forebrain excitatory neurons on behavioural correlates of mood disorders. Eur Arch Psychiatry Clin Neurosci 264: 625–629.
von Bohlen und Halbach O, Zacher C, Gass P, Unsicker K (2006). Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res 83: 525–531.
Weber T, Bohm G, Hermann E, Schutz G, Schonig K, Bartsch D (2009). Inducible gene manipulations in serotonergic neurons. Front Mol Neurosci 2: 24.
Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K et al (2008). Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and 'schizophrenia-related' behaviors. Mol Psychiatry 13: 631–640.
Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A et al (1999). Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811.
Zill P, Baghai TC, Zwanzger P, Schule C, Eser D, Rupprecht R et al (2004). SNP and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene provide evidence for association with major depression. Mol Psychiatry 9: 1030–1036.
Acknowledgements
We kindly thank Professor Rohini Kuner (University Heidelberg) for the help with measurements of the tail flick latency. We kindly thank Lena Wendler and Christiane Brandwein for their help with animal care, revitalization, perfusions, tamoxifen injections, and genotyping.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Weber, T., Vogt, M., Gartside, S. et al. Adult AMPA GLUA1 Receptor Subunit Loss in 5-HT Neurons Results in a Specific Anxiety-Phenotype with Evidence for Dysregulation of 5-HT Neuronal Activity. Neuropsychopharmacol 40, 1471–1484 (2015). https://doi.org/10.1038/npp.2014.332
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.332
This article is cited by
-
Generalized and social anxiety disorder interactomes show distinctive overlaps with striosome and matrix interactomes
Scientific Reports (2021)
-
Fetal glucocorticoid receptor (Nr3c1) deficiency alters the landscape of DNA methylation of murine placenta in a sex-dependent manner and is associated to anxiety-like behavior in adulthood
Translational Psychiatry (2019)