Abstract
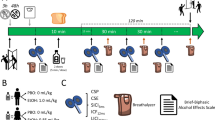
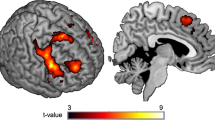
Alcohol is a rich drug affecting both the γ-amino butyric acid (GABA) and glutamatergic neurotransmitter systems. Recent findings from both modeling and pharmacological manipulation have indicated a link between GABAergic activity and oscillations measured in the gamma frequency range (30–80 Hz), but there are no previous reports of alcohol’s modulation of gamma-band activity measured by magnetoencephalography (MEG) or electroencephalography (EEG). In this single-blind, placebo-controlled crossover study, 16 participants completed two study days, on one day of which they consumed a dose of 0.8 g/kg alcohol, and on the other day a placebo. MEG recordings of brain activity were taken before and after beverage consumption, using visual grating and finger abduction paradigms known to induce gamma-band activity in the visual and motor cortices respectively. Time–frequency analyses of beamformer source reconstructions in the visual cortex showed that alcohol increased peak gamma amplitude and decreased peak frequency. For the motor task, alcohol increased gamma amplitude in the motor cortex. These data support the notion that gamma oscillations are dependent, in part, on the balance between excitation and inhibition. Disruption of this balance by alcohol, by increasing GABAergic inhibition at GABAA receptors and decreasing glutamatergic excitation at N-methyl-D-aspartic acid receptors, alters both the amplitude and frequency of gamma oscillations. The findings provide further insight into the neuropharmacological action of alcohol.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahveninen J, Lin F, Kivisaari R, Autti T, Hämäläinen M, Stufflebeam S et al (2007). MRI-constrained spectral imaging of benzodiazepine modulation of spontaneous neuromagnetic activity in human cortex. NeuroImage 35: 577–582.
Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Health Care 2nd edn, World Health Organisation: Geneva, Switzerland.
Ball DM, Glue P, Wilson S, Nutt DJ, Unit CP (1991). Pharmacology of saccadic eye movements in man 1. Psychopharmacology (Berl) 105: 361–367.
Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C (1994). Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol 269: 219–224.
Brainard DH (1997). The psychophysics toolbox. Spat Vis 10: 433–436.
Brumback T, Cao D, King A (2007). Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend 91: 10–17.
Buzsáki G, Wang X-J (2012). Mechanisms of gamma oscillations. Annu Rev Neurosci 35: 203–225.
Carl C, Açık A, König P, Engel AK, Hipp JF (2012). The saccadic spike artefact in MEG. NeuroImage 59: 1657–1667.
Castelo-Branco M, Neuenschwander S, Singer W (1998). Synchronization of visual responses between the cortex, lateral geniculate nucleus, and retine in the anesthetized cat. J Neucosci 18: 6395–6410.
Cheyne D, Bells S, Ferrari P, Gaetz W, Bostan AC (2008). Self-paced movements induce high-frequency gamma oscillations in primary motor cortex. Neuroimage 42: 332–342.
Gaetz W, Roberts TPL, Singh KD, Muthukumaraswamy SD (2012). Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp 33: 2035–2046.
Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G et al (2012). Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry 71: 239–246.
Gonzalez-Burgos G, Lewis D (2008). GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull 34: 944–961.
Grant KA, Lovinger DM (1995). Cellular and behavioural neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci 3: 155–164.
Hall SD, Barnes GR, Furlong PL, Seri S, Hillebrand A (2010). Neuronal network pharmacodynamics of GABAergic modulation in the human cortex determined using pharmaco-magnetoencephalography. Hum Brain Mapp 31: 581–594.
Hamandi K, Singh KD, Muthukumaraswamy S (2011). Reduced movement-related β desynchronisation in juvenile myoclonic epilepsy: a MEG study of task specific cortical modulation. Clin Neurophysiol 122: 2128–2138.
Jenkinson M, Smith S (2001). A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156.
Jensen O, Goel P, Kopell N, Pohja M, Hari R, Ermentrout B (2005). On the human sensorimotor-cortex beta rhythm: sources and modeling. Neuroimage 26: 347–355.
Jurkiewicz MT, Gaetz WC, Bostan AC, Cheyne D (2006). Post-movement beta rebound is generated in motor cortex: evidence from neuromagnetic recordings. Neuroimage 32: 1281–1289.
Kahlbrock N, Butz M, May ES, Schnitzler A (2012). Sustained gamma band synchronization in early visual areas reflects the level of selective attention. Neuroimage 59: 673–681.
Koelewijn L, Rich AN, Muthukumaraswamy SD, Singh KD (2013). Spatial attention increases high-frequency gamma synchronisation in human medial visual cortex. Neuroimage 79: 295–303.
Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K (2012). Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One 7: e43957.
Lehtinen I, Lang AH, Jäntti V, Keskinen E (1979). Acute effects of alcohol on saccadic eye movements. Psychopharmacolgy 63: 17–23.
Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, Foltynie T et al (2012). Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simulatneous megnetoencephalography and intracranial recordings. J Neurosci 32: 10541–10553.
Lovinger DM, White G, Weight FF (1989). Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science 243: 1721–1724.
Lovinger DM, White G, Weight FF (1990). NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci 10: 1372–1379.
Marinkovic K, Rosen BQ, Cox B, Kovacevic S (2012). Event-related theta power during lexical-semantic retrieval and decision conflict is modulated by alcohol intoxication: anatomically constrained MEG. Front Psychol 3: 121.
Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM (1993). Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res 17: 140–146.
Muthukumaraswamy SD (2010). Functional properties of human primary motor cortex gamma oscillations. J Neurophysiol 104: 2873–2885.
Muthukumaraswamy SD, Edden RAE, Jones DK, Swettenham JB, Singh KD (2009). Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci USA 106: 8356–8361.
Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Hamandi K, Lingford-Hughes A et al (2013a). Elevating endogenous GABA levels with GAT-1 blockade modulates evoked but not induced responses in human visual cortex. Neuropsychopharmacology 1: 1–8.
Muthukumaraswamy SD, Myers JFM, Wilson SJ, Nutt DJ, Lingford-Hughes A, Singh KD et al (2013b). The effects of elevated endogenous GABA levels on movement-related network oscillations. Neuroimage 66: 36–41.
Muthukumaraswamy SD, Singh KD (2013). Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage 69: 223–230.
Nikulin VV, Nikulina AV, Yamashita H, Rossi EM, Kähkönen S (2005). Effects of alcohol on spontaneous neuronal oscillations: a combined magnetoencephalography and electroencephalography study. Prog Neuro-Psychopharmacol Biol Psychiatry 29: 687–693.
Nutt DJ, Besson M, Wilson SJ, Dawson GR, Lingford-Hughes AR (2007). Blockade of alcohol’s amnestic activity in humans by an alpha5 subtype benzodiazepine receptor inverse agonist. Neuropharmacology 53: 810–820.
Oke OO, Magony A, Anver H, Ward PD, Jiruska P, Jefferys JGR et al (2010). High-frequency gamma oscillations coexist with low-frequency gamma oscillations in the rat visual cortex in vitro. Eur J Neurosci 31: 1435–1445.
Orser A, Pennefather PS, Macdonald JF (1994). Propofol modulates activation and desensitization of GABA, receptors in cultured murine hippocampal neurons. J Neurosci 74: 7747–7760.
Pelli DG (1997). The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis 10: 437–442.
Pfurtscheller G, Lopes da Silva FH (1999). Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110: 1842–1857.
Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR (2003). Ethanol increases GABAergic transmission at both pre- and post-synaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA 100: 2053–2058.
Robinson SE, Vrba J (1999). Functional neuroimaging by synthetic aperture manetometry (SAM). In: Yoshimoto T, Kotani M, Kuriki S, Karibe H, Nakasato N (Eds.) Recent Advances in Biomagnetism, Tohoku University Press: Sendai. pp 302–305.
Rose AK, Duka T (2008). Effects of alcohol on inhibitory processes. Behav Pharmacol 19: 284–291.
Saxena N, Muthukumaraswamy SD, Diukova A, Singh K, Hall J, Wise R (2013). Enhanced stimulus-induced gamma activity in humans during propofol-induced sedation. PLoS One 8: e57685.
Schuckit MA (1980). Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol 41: 242–249.
Singer W (1993). Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol 55: 349–374.
Stockwell T, Murphy D, Hodgson R (1983). Severity of alcohol dependence questionnaire (SADQ). Br J Addict 78: 45–156.
Sutker PB, Tabakoff B, Goist KC, Randall CL (1983). Acute alcohol intoxication, mood states and alcohol metabolism in women and men. Pharmacol Biochem Behav 18 (Suppl 1): 349–354.
Swettenham JB, Muthukumaraswamy SD, Singh KD (2009). Spectral properties of induced and evoked gamma oscillations in human early visual cortex to moving and stationary stimuli. J Neurophysiol 102: 1241–1253.
Valenzuela CF (1997). Alcohol and neurotransmitter interactions. Alcohol Health Res World 21: 144–148.
Van Quyen M, Le, Foucher J, Lachaux J, Rodriguez E, Lutz A, Martinerie J et al (2001). Comparison of Hilbert transform and wavelet methods for the analysis of neuronal synchrony. J Neurosci Methods 111: 83–98.
Vrba J, Robinson SE (2001). Signal processing in magnetoencephalography. Methods 25: 249–271.
Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR (1996). Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci USA 93: 5049–5054.
Weiner JL, Valenzuela CF (2006). Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther 111: 533–554.
Zigmond AS, Snaith RP (1983). The hospital anxiety and depression scale. Acta Psychiatr Scand 37: 361–370.
Acknowledgements
Special thanks to all who assisted with the running of the study, especially Alex Shaw, Jenny Brealy, and Kacper Wieczorek for assisting with data collection, Anne Lingford-Hughes and Angela Attwood for advice on alcohol administration and breathalyzers, and Geoffrey Mégardon and Brice Dassy for their contribution to developing the Gaussian function fits. Anne Campbell receives a studentship funded by Alcohol Research UK and Cardiff University School of Psychology. This study was supported by CUBRIC and the School of Psychology at Cardiff University, together with the MRC/EPSRC funded UK MEG Partnership Grant (MR/K005464/1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Campbell, A., Sumner, P., Singh, K. et al. Acute Effects of Alcohol on Stimulus-Induced Gamma Oscillations in Human Primary Visual and Motor Cortices. Neuropsychopharmacol 39, 2104–2113 (2014). https://doi.org/10.1038/npp.2014.58
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2014.58
This article is cited by
-
No effects of transcranial direct current stimulation on visual evoked potential and peak gamma frequency
Cognitive Processing (2022)
-
Genetic risk for schizophrenia is associated with altered visually-induced gamma band activity: evidence from a population sample stratified polygenic risk
Translational Psychiatry (2021)
-
Visual gamma oscillations predict sensory sensitivity in females as they do in males
Scientific Reports (2021)
-
Effects of Acute Ethanol Intoxication on Local Field Potentials in the Rat Lateral Septum
Neurophysiology (2021)
-
Region-dependent regulation of acute ethanol on γ oscillation in the rat hippocampal slices
Psychopharmacology (2020)