Abstract
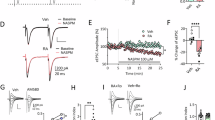
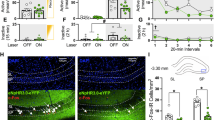
The ubiquitin-proteasome system (UPS) has been implicated in the retrieval-induced destabilization of cocaine- and fear-related memories in Pavlovian paradigms. However, nothing is known about its role in memory retrieval after self-administration of cocaine, an operant paradigm, or how the length of withdrawal from cocaine may influence retrieval mechanisms. Here, we examined UPS activity after an extended-access cocaine self-administration regimen that leads to withdrawal-dependent incubation of cue-induced cocaine craving. Controls self-administered saline. In initial experiments, memory retrieval was elicited via a cue-induced seeking/retrieval test on withdrawal day (WD) 50–60, when craving has incubated. We found that retrieval of cocaine- and saline-associated memories produced similar increases in polyubiquitinated proteins in the nucleus accumbens (NAc), compared with rats that did not undergo a seeking/retrieval test. Measures of proteasome catalytic activity confirmed similar activation of the UPS after retrieval of saline and cocaine memories. However, in a subsequent experiment in which testing was conducted on WD1, proteasome activity in the NAc was greater after retrieval of cocaine memory than saline memory. Analysis of other brain regions confirmed that effects of cocaine memory retrieval on proteasome activity, relative to saline memory retrieval, depend on withdrawal time. These results, combined with prior studies, suggest that the relationship between UPS activity and memory retrieval depends on training paradigm, brain region, and time elapsed between training and retrieval. The observation that mechanisms underlying cocaine memory retrieval change depending on the age of the memory has implications for development of memory destabilization therapies for cue-induced relapse in cocaine addicts.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bailey J, Ma D, Szumlinski KK (2012). Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol 17: 248–258.
Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE et al (2013). Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci 33: 495–506.
Bingol B, Sheng M (2011). Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron 69: 22–32.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Ehlers MD (2003). Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci 6: 231–242.
Fan HY, Cherng CG, Yang FY, Cheng LY, Tsai CJ, Lin LC et al (2010). Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav Brain Res 208: 522–527.
Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME (2010). The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology 35: 818–833.
Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nägerl UV (2006). A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron 52: 239–245.
Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ (2006). Stability of recent and remote contextual fear memory. Learning Mem 13: 451–457.
Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI (2009). Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci 30: 889–900.
Fuchs RA, Branham RK, See RE (2006). Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26: 3584–3588.
Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y (2003). Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci 23: 742–747.
Hanley JG (2014). Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca2+-permeable AMPA receptors. Semin Cell Dev Biol 27: 14–22.
Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ (2011). Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS ONE 6: e24349.
Jarome TJ, Kwapis JL, Ruenzel WL, Helmstetter FJ (2013). CaMKII, but not protein kinase A, regulates Rpt6 phosphorylation and proteasome activity during the formation of long-term memories. Front Behav Neurosci 7: 115.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009). Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56: 177–185.
Lee JL, Milton AL, Everitt BJ (2006). Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26: 5881–5887.
Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK et al (2008). Synaptic protein degradation underlies destabilization of retrieved fear memory. Science 319: 1253–1256.
Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M et al (2013). Maturation of silent synapses in amygdala-accumbens projections contributes to incubation of cocaine craving. Nat Neurosci 16: 1644–1651.
Li AW, Man HY (2013). Ubiquitination of neurotransmitter receptors and postsynaptic scaffolding proteins. Neural Plast 2013: 432057.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT et al (2014). Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 17: 73–80.
Lu L, Grimm JW, Hope BT, Shaham Y (2004). Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47: 214–226.
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005a). Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8: 212–219.
Lu L, Dempsey J, Shaham Y, Hope BT (2005b). Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem 94: 161–168.
Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y (2007). Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Pyschiatry 61: 591–598.
Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R et al (2014). Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83: 1453–1467.
Massaly N, Dahan L, Baudonnat M, Hovnanian C, Rekik K, Solinas M et al (2013). Involvement of protein degradation by the ubiquitin proteasome system in opiate addictive behaviors. Neuropsychopharmacology 38: 596–604.
McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M (2011). Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration by not experimenter-administered cocaine. J Neurosci 31: 5737–5743.
Milekic MH, Alberini CM (2002). Temporally graded requirement for protein synthesis following memory reactivation. Neuron 36: 521–525.
Milton AL, Everitt BJ (2010). The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci 31: 2308–2319.
Mogenson GJ, Jones DL, Yim CY (1980). From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97.
Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J et al (2008). Ubiquitin chain editing revealed by polyubiquitinated linkage-specific antibodies. Cell 134: 668–678.
Pacchioni AM, Gabriele A, See RE (2011). Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res 218: 296–300.
Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509: 459–464.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011). Neurobiology of the incubation of drug craving. Trends Neurosci 34: 411–420.
Ren ZY, Liu MM, Xue YX, Ding ZB, Xue LF, Zhai SD et al (2013). A critical role for protein degradation in the nucleus accumbens core in cocaine reward memory. Neuropsychopharmacology 38: 778–790.
See RE, Elliott JC, Feltenstein MW (2007). The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology 194: 321–331.
Shen H, Korutla L, Champtiaux N, Toda S, LaLumiere R, Vallone J et al (2007). NAC1 regulates the recruitment of the proteasome complex into dendritic spines. J Neurosci 27: 8903–8913.
Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM (2014). Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology 231: 3109–3118.
Théberge FR, Milton AL, Belin D, Lee JL, Everitt BJ (2010). The basolateral amygdala and nucleus accumbens core mediate dissociable aspects of drug memory reconsolidation. Learn Mem 17: 444–453.
Torregrossa MM, Taylor JR (2013). Learning to forget: manipulating extinction and reconsolidation processes to treat addiction. Psychopharmacology 226: 659–672.
Wolf ME, Tseng KY (2012). Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci 5: 72.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Werner, C., Milovanovic, M., Christian, D. et al. Response of the Ubiquitin-Proteasome System to Memory Retrieval After Extended-Access Cocaine or Saline Self-Administration. Neuropsychopharmacol 40, 3006–3014 (2015). https://doi.org/10.1038/npp.2015.156
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.156
This article is cited by
-
Neuroadaptations and TGF-β signaling: emerging role in models of neuropsychiatric disorders
Molecular Psychiatry (2022)
-
Ube2b-dependent degradation of DNMT3a relieves a transcriptional brake on opiate-induced synaptic and behavioral plasticity
Molecular Psychiatry (2021)
-
Genome-wide transcriptional profiling of central amygdala and orbitofrontal cortex during incubation of methamphetamine craving
Neuropsychopharmacology (2018)