Abstract
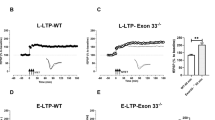
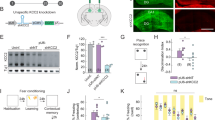
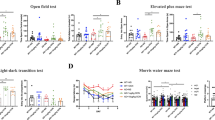
Calsyntenin-2 has an evolutionarily conserved role in cognition. In a human genome-wide screen, the CLSTN2 locus was associated with verbal episodic memory, and expression of human calsyntenin-2 rescues the associative learning defect in orthologous Caenorhabditis elegans mutants. Other calsyntenins promote synapse development, calsyntenin-1 selectively of excitatory synapses and calsyntenin-3 of excitatory and inhibitory synapses. We found that targeted deletion of calsyntenin-2 in mice results in a selective reduction in functional inhibitory synapses. Reduced inhibitory transmission was associated with a selective reduction of parvalbumin interneurons in hippocampus and cortex. Clstn2−/− mice showed normal behavior in elevated plus maze, forced swim test, and novel object recognition assays. However, Clstn2−/− mice were hyperactive in the open field and showed deficits in spatial learning and memory in the Morris water maze and Barnes maze. These results confirm a function for calsyntenin-2 in cognitive performance and indicate an underlying mechanism that involves parvalbumin interneurons and aberrant inhibitory transmission.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Araki Y, Kawano T, Taru H, Saito Y, Wada S, Miyamoto K et al (2007). The novel cargo Alcadein induces vesicle association of kinesin-1 motor components and activates axonal transport. Embo J 26: 1475–1486.
Araki Y, Miyagi N, Kato N, Yoshida T, Wada S, Nishimura M et al (2004). Coordinated metabolism of Alcadein and amyloid beta-protein precursor regulates FE65-dependent gene transactivation. J Biol Chem 279: 24343–24354.
Barnes CA (1979). Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol 93: 74–104.
Brielmaier J, Matteson PG, Silverman JL, Senerth JM, Kelly S, Genestine M et al (2012). Autism-relevant social abnormalities and cognitive deficits in engrailed-2 knockout mice. PLoS One 7: e40914.
Cohen SJ, Stackman RW Jr (2015). Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav Brain Res 285: 105–117.
Donato F, Rompani SB, Caroni P (2013). Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature 504: 272–276.
Hales JB, Schlesiger MI, Leutgeb JK, Squire LR, Leutgeb S, Clark RE (2014). Medial entorhinal cortex lesions only partially disrupt hippocampal place cells and hippocampus-dependent place memory. Cell Rep 9: 893–901.
Han S, Tai C, Jones CJ, Scheuer T, Catterall WA (2014). Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron 81: 1282–1289.
Harrison FE, Hosseini AH, McDonald MP (2009). Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res 198: 247–251.
Hintsch G, Zurlinden A, Meskenaite V, Steuble M, Fink-Widmer K, Kinter J et al (2002). The calsyntenins—a family of postsynaptic membrane proteins with distinct neuronal expression patterns. Mol Cell Neurosci 21: 393–409.
Hoerndli FJ, Walser M, Frohli Hoier E, de Quervain D, Papassotiropoulos A, Hajnal A (2009). A conserved function of C. elegans CASY-1 calsyntenin in associative learning. PLoS ONE 4: e4880.
Hu H, Gan J, Jonas P (2014). Interneurons. Fast-spiking, parvalbumin(+) GABAergic interneurons: from cellular design to microcircuit function. Science 345: 1255263.
Ikeda DD, Duan Y, Matsuki M, Kunitomo H, Hutter H, Hedgecock EM et al (2008). CASY-1, an ortholog of calsyntenins/alcadeins, is essential for learning in Caenorhabditis elegans. Proc Natl Acad Sci USA 105: 5260–5265.
Jacobsen LK, Picciotto MR, Heath CJ, Mencl WE, Gelernter J (2009). Allelic variation of calsyntenin 2 (CLSTN2) modulates the impact of developmental tobacco smoke exposure on mnemonic processing in adolescents. Biol Psychiatry 65: 671–679.
Kohannim O, Hibar DP, Stein JL, Jahanshad N, Hua X, Rajagopalan P et al (2012). Discovery and replication of gene influences on brain structure using LASSO regression. Front Neurosci 6: 115.
Konecna A, Frischknecht R, Kinter J, Ludwig A, Steuble M, Meskenaite V et al (2006). Calsyntenin-1 docks vesicular cargo to kinesin-1. Mol Biol Cell 17: 3651–3663.
Laukka EJ, Lovden M, Herlitz A, Karlsson S, Ferencz B, Pantzar A et al (2013). Genetic effects on old-age cognitive functioning: a population-based study. Psychol Aging 28: 262–274.
Marin O (2012). Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 13: 107–120.
Morris RG, Garrud P, Rawlins JN, O'Keefe J (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683.
Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ et al (2009). Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol 7: e41.
Pantzar A, Laukka EJ, Atti AR, Papenberg G, Keller L, Graff C et al (2014). Interactive effects of KIBRA and CLSTN2 polymorphisms on episodic memory in old-age unipolar depression. Neuropsychologia 62C: 137–142.
Papassotiropoulos A, Stephan DA, Huentelman MJ, Hoerndli FJ, Craig DW, Pearson JV et al (2006). Common Kibra alleles are associated with human memory performance. Science 314: 475–478.
Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H et al (2011). Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 147: 235–246.
Pettem KL, Yokomaku D, Luo L, Linhoff MW, Prasad T, Connor SA et al (2013). The specific alpha-neurexin interactor calsyntenin-3 promotes excitatory and inhibitory synapse development. Neuron 80: 113–128.
Preuschhof C, Heekeren HR, Li SC, Sander T, Lindenberger U, Backman L (2010). KIBRA and CLSTN2 polymorphisms exert interactive effects on human episodic memory. Neuropsychologia 48: 402–408.
Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H et al (1999). Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci 2: 898–905.
Saab BJ, Saab AM, Roder JC (2011). Statistical and theoretical considerations for the platform re-location water maze. J Neurosci Methods 198: 44–52.
Sgado P, Genovesi S, Kalinovsky A, Zunino G, Macchi F, Allegra M et al (2013). Loss of GABAergic neurons in the hippocampus and cerebral cortex of Engrailed-2 null mutant mice: implications for autism spectrum disorders. Exp Neurol 247: 496–505.
Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ et al (2012). The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci 32: 2988–2997.
Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A (2003). Performance of different mouse strains in an object recognition task. Behav Brain Res 147: 49–54.
Somogyi P, Klausberger T (2005). Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562: 9–26.
Ster J, Steuble M, Orlando C, Diep TM, Akhmedov A, Raineteau O et al (2014). Calsyntenin-1 regulates targeting of dendritic NMDA receptors and dendritic spine maturation in CA1 hippocampal pyramidal cells during postnatal development. J Neurosci 34: 8716–8727.
Van Cauter T, Camon J, Alvernhe A, Elduayen C, Sargolini F, Save E (2013). Distinct roles of medial and lateral entorhinal cortex in spatial cognition. Cereb Cortex 23: 451–459.
Won H, Mah W, Kim E, Kim JW, Hahm EK, Kim MH et al (2011). GIT1 is associated with ADHD in humans and ADHD-like behaviors in mice. Nat Med 17: 566–572.
Acknowledgements
We thank Nazarine Fernandes for excellent technical assistance and Konstantin Pavlov for skillful assistance with EthoVision.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Lipina, T., Prasad, T., Yokomaku, D. et al. Cognitive Deficits in Calsyntenin-2-deficient Mice Associated with Reduced GABAergic Transmission. Neuropsychopharmacol 41, 802–810 (2016). https://doi.org/10.1038/npp.2015.206
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.206
This article is cited by
-
Comprehensive analyses of circulating cardiometabolic proteins and objective measures of fat mass
International Journal of Obesity (2023)
-
Loss of calsyntenin paralogs disrupts interneuron stability and mouse behavior
Molecular Brain (2022)
-
Perinatal Lead Exposure Alters Calsyntenin-2 and Calsyntenin-3 Expression in the Hippocampus and Causes Learning Deficits in Mice Post-weaning
Biological Trace Element Research (2021)
-
Hippocampal µ-opioid receptors on GABAergic neurons mediate stress-induced impairment of memory retrieval
Molecular Psychiatry (2020)
-
Inhibition in the amygdala anxiety circuitry
Experimental & Molecular Medicine (2018)