Abstract
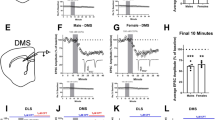
The striatum has an essential role in neural control of instrumental behaviors by reinforcement learning. Adenosine A2A receptors (A2ARs) are highly enriched in the striatopallidal neurons and are implicated in instrumental behavior control. However, the temporal importance of the A2AR signaling in relation to the reward and specific contributions of the striatopallidal A2ARs in the dorsolateral striatum (DLS) and the dorsomedial striatum (DMS) to the control of instrumental learning are not defined. Here, we addressed temporal relationship and sufficiency of transient activation of optoA2AR signaling precisely at the time of the reward to the control of instrumental learning, using our newly developed rhodopsin-A2AR chimeras (optoA2AR). We demonstrated that transient light activation of optoA2AR signaling in the striatopallidal neurons in ‘time-locked’ manner with the reward delivery (but not random optoA2AR activation) was sufficient to change the animal’s sensitivity to outcome devaluation without affecting the acquisition or extinction phases of instrumental learning. We further demonstrated that optogenetic activation of striatopallidal A2AR signaling in the DMS suppressed goal-directed behaviors, as focally genetic knockdown of striatopallidal A2ARs in the DMS enhanced goal-directed behavior by the devaluation test. By contrast, optogenetic activation or focal AAV-Cre-mediated knockdown of striatopallidal A2AR in the DLS had relatively limited effects on instrumental learning. Thus, the striatopallidal A2AR signaling in the DMS exerts inhibitory and predominant control of goal-directed behavior by acting precisely at the time of reward, and may represent a therapeutic target to reverse abnormal habit formation that is associated with compulsive obsessive disorder and drug addiction.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abeliovich A, Gerber D, Tanaka O, Katsuki M, Graybiel AM, Tonegawa S (1992). On somatic recombination in the central nervous system of transgenic mice. Science 257: 404–410.
Aquili L, Liu AW, Shindou M, Shindou T, Wickens JR (2014). Behavioral flexibility is increased by optogenetic inhibition of neurons in the nucleus accumbens shell during specific time segments. Learn Mem 21: 223–231.
Augusto E, Matos M, Sevigny J, El-Tayeb A, Bynoe MS, Muller CE et al (2013). Ecto-5'-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci 33: 11390–11399.
Balleine BW, Liljeholm M, Ostlund SB (2009). The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res 199: 43–52.
Brainard MS, Doupe AJ (2000). Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature 404: 762–766.
Brown Gould B, Graybiel AM (2010). Afferents to the cerebellar cortex in the cat: evidence for an intrinsic pathway leading from the deep nuclei to the cortex. 1976. Cerebellum 9: 1–13.
Burguiere E, Monteiro P, Feng G, Graybiel AM (2013). Optogenetic stimulation of lateral orbitofronto-striatal pathway suppresses compulsive behaviors. Science 340: 1243–1246.
Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR et al (2003). Adenosine A2A-dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741–46749.
Chen JF (2014). Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol 119: 257–307.
Chen JF, Eltzschig HK, Fredholm BB (2013). Adenosine receptors as drug targets—what are the challenges? Nat Rev Drug Discov 12: 265–286.
Derusso AL, Fan D, Gupta J, Shelest O, Costa RM, Yin HH (2010). Instrumental uncertainty as a determinant of behavior under interval schedules of reinforcement. Front Integr Neurosci 4: 17 (doi: 10.3389/fnint.2010.00017).
Desmurget M, Turner RS (2010). Motor sequences and the basal ganglia: kinematics, not habits. J Neuroscience 30: 7685–7690.
Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M et al (2009). D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci 12: 393–395.
Durieux PF, Schiffmann SN, de Kerchove d'Exaerde A (2012). Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J 31: 640–653.
Ferre S, Karcz-Kubicha M, Hope BT, Popoli P, Burgueno J, Gutierrez MA et al (2002). Synergistic interaction between adenosine A2A and glutamate mGlu5 receptors: implications for striatal neuronal function. Proc Natl Acad Sci USA 99: 11940–11945.
Gillan CM, Papmeyer M, Morein-Zamir S, Sahakian BJ, Fineberg NA, Robbins TW et al (2011). Disruption in the balance between goal-directed behavior and habit learning in obsessive-compulsive disorder. Am J Psychiatry 168: 718–726.
Gremel CM, Costa RM (2013). Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual actions. Nat Commun 4: 2264.
Higley MJ, Sabatini BL (2010). Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13: 958–966.
Histed MH, Pasupathy A, Miller EK (2009). Learning substrates in the primate prefrontal cortex and striatum: sustained activity related to successful actions. Neuron 63: 244–253.
Knowlton BJ, Mangels JA, Squire LR (1996). A neostriatal habit learning system in humans. Science 273: 1399–1402.
Lawrence AD, Sahakian BJ, Robbins TW (1998). Cognitive functions and corticostriatal circuits: insights from Huntington’s disease. Trends Cogn Sci 2: 379–388.
Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE et al (2011). Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 31: 10067–10075.
Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X et al (2015). Optogenetic activation of intracellular adenosine A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry 1–11 (doi:10.1038/mp.2014.182).
Lovinger DM (2010). Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology 58: 951–961.
Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM et al (2008). Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci 28: 9037–9046.
Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A et al (2013). Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci 33: 4329–4338.
Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M et al (2012). SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci 15: 284–293.
Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD (2013). Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev 37 (Part A): 2015–2025.
Ostlund SB, Balleine BW (2008). On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models 5: 235–245.
Pomata PE, Belluscio MA, Riquelme LA, Murer MG (2008). NMDA receptor gating of information flow through the striatum in vivo. J Neurosci 28: 13384–13389.
Redgrave P, Rodriguez M, Smith Y, Rodriguez-Oroz MC, Lehericy S, Bergman H et al (2010). Goal-directed and habitual control in the basal ganglia: implications for Parkinson's disease. Nat Rev Neurosci 11: 760–772.
Reynolds JN, Hyland BI, Wickens JR (2001). A cellular mechanism of reward-related learning. Nature 413: 67–70.
Rossi MA, Sukharnikova T, Hayrapetyan VY, Yang L, Yin HH (2013). Operant self-stimulation of dopamine neurons in the substantia nigra. PLoS One 8: e65799.
Schultz W, Dayan P, Montague PR (1997). A neural substrate of prediction and reward. Science 275: 1593–1599.
Shen HY, Canas PM, Garcia-Sanz P, Lan JQ, Boison D, Moratalla R et al (2013). Adenosine A(2)A receptors in striatal glutamatergic terminals and GABAergic neurons oppositely modulate psychostimulant action and DARPP-32 phosphorylation. PLoS One 8: e80902.
Singer P, Wei CJ, Chen JF, Boison D, Yee BK (2013). Deletion of striatal adenosine A(2A) receptor spares latent inhibition and prepulse inhibition but impairs active avoidance learning. Behav Brain Res 242: 54–61.
Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH (2013). A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci 16: 966–973.
Svenningsson P, Le Moine C, Fisone G, Fredholm BB (1999). Distribution, biochemistry and function of striatal adenosine A2A receptors. Progr Neurobiol 59: 355–396.
Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L (2012). Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci 15: 1281–1289.
Thorn CA, Atallah H, Howe M, Graybiel AM (2010). Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66: 781–795.
Wei CJ, Augusto E, Gomes CA, Singer P, Wang Y, Boison D et al (2014). Regulation of fear responses by striatal and extrastriatal adenosine A2A receptors in forebrain. Biol Psychiatry 75: 855–863.
Wei CJ, Singer P, Coelho J, Boison D, Feldon J, Yee BK et al (2011). Selective inactivation of adenosine A(2A) receptors in striatal neurons enhances working memory and reversal learning. Learn Mem 18: 459–474.
Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S, Kasai H (2014). A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345: 1616–1620.
Yin HH, Knowlton BJ (2006). The role of the basal ganglia in habit formation. Nat Rev Neurosci 7: 464–476.
Yin HH, Ostlund SB, Balleine BW (2008). Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci 28: 1437–1448.
Yu C, Gupta J, Chen JF, Yin HH (2009). Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci 29: 15100–15103.
Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT et al (2009). Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res 1303: 74–83.
Acknowledgements
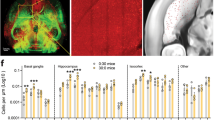
We thank Liu Ya for her assistance in image analysis of optical fluorescent density.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Li, Y., He, Y., Chen, M. et al. Optogenetic Activation of Adenosine A2A Receptor Signaling in the Dorsomedial Striatopallidal Neurons Suppresses Goal-Directed Behavior. Neuropsychopharmacol 41, 1003–1013 (2016). https://doi.org/10.1038/npp.2015.227
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.227
This article is cited by
-
Neuronal and astrocytic CB1R signaling differentially modulates goal-directed behavior and working memory by distinct temporal mechanisms
Neuropsychopharmacology (2023)
-
The role of dorsomedial striatum adenosine 2A receptors in the loss of goal-directed behaviour
Psychopharmacology (2023)
-
DNA methylation and hydroxymethylation characterize the identity of D1 and D2 striatal projection neurons
Communications Biology (2022)
-
Towards translational optogenetics
Nature Biomedical Engineering (2022)
-
A Translation from Goal-Directed to Habitual Control: the Striatum in Drug Addiction
Current Addiction Reports (2021)