Abstract
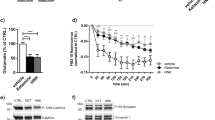
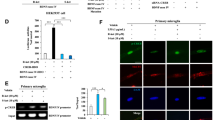
We have reported the antidepressant effects of both metabotropic glutamate 2/3 (mGlu2/3) receptor antagonists and ketamine in several animal models, and proposed that serotonergic (5-HTergic) transmission is involved in these actions. Given that the projections from the medial prefrontal cortex (mPFC) to the dorsal raphe nucleus (DRN), where the majority of serotonin (5-HT) neurons exist, are reportedly involved in the antidepressant effects, in this study, we investigated using the forced swimming test (FST) of C57BL/6J male mice, the role of 5-HT neurons in the DRN regulated by the mPFC–DRN projections in the antidepressant effects of an mGlu2/3 receptor antagonist, LY341495, and ketamine. Following systemic administration/microinjection into the mPFC, both LY341495 and ketamine were found to exert antidepressant effects in the FST, and the effects were attenuated by depletion of 5-HT by treatment with an inhibitor of 5-HT synthesis, PCPA. The antidepressant effects of LY341495 and ketamine were also blocked by systemic administration/microinjection into the mPFC of an AMPA receptor antagonist, NBQX. Moreover, systemic administration/microinjection into the mPFC of LY341495 and ketamine significantly increased the c-Fos expression in the 5-HT neurons in the DRN, and the effect of systemic administration of these drugs on the neuronal c-Fos expression was attenuated by microinjection of NBQX into the mPFC. Our findings suggest that activation of 5-HT neurons in the DRN regulated by stimulation of the AMPA receptor in the mPFC may be involved in the antidepressant effects of an mGlu2/3 receptor antagonist and ketamine.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ago Y, Yano K, Araki R, Hiramatsu N, Kita Y, Kawasaki T et al (2013). Metabotropic glutamate 2/3 receptor antagonists improve behavioral and prefrontal dopaminergic alterations in the chronic corticosterone-induced depression model in mice. Neuropharmacology 65: 29–38.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF et al (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91–95.
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS (2000). Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351–354.
Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N et al (2004). MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant like activity. Neuropharmacology 46: 457–467.
Challis C, Beck SG, Berton O (2014). Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci 8: 43.
Dwyer JM, Lepack AE, Duman RS (2012). mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol 15: 429–434.
Dwyer JM, Lepack AE, Duman RS (2013). mGluR2/3 blockade produces rapid and long-lasting reversal of anhedonia caused by chronic stress exposure. J Mol Psychiatry 1: 15.
Fukumoto K, Iijima M, Chaki S (2014). Serotonin-1A receptor stimulation mediates effects of a metabotropic glutamate 2/3 receptor antagonist, 2S-2-amino-2-(1S,2S-2-carboxycycloprop-1-yl)-3-(xanth-9-yl)propanoic acid (LY341495), and an N-methyl-D-aspartate receptor antagonist, ketamine, in the novelty-suppressed feeding test. Psychopharmacology (Berl) 2231: 2291–2298.
Gigliucci V, O'Dowd G, Casey S, Egan D, Gibney S, Harkin A (2013). Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology (Berl) 228: 157–166.
Gradient RA, Wedel PC, Frisbie VM, Leuchter AF, Targum SD, Truong CT et al (2012). Safety, pharmacokinetic and pharmacodynamics profile of BCI-632, a selective metabotropic glutamate 2/3 receptor antagonist, in healthy human subjects. Abstr Neurosci Meeting 42: 20.
Hamani C, Diwan M, Macedo CE, Brandão ML, Shumake J, Gonzalez-Lima F et al (2010). Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol Psychiatry 67: 117–124.
Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A et al (2010). Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem 115: 1608–1620.
Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG et al (2011). Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry 35: 1155–1159.
Iijima M, Koike H, Chaki S (2013). Effect of an mGlu2/3 receptor antagonist on depressive behavior induced by withdrawal from chronic treatment with methamphetamine. Behav Brain Res 246: 24–28.
Jankowski MP, Sesack SR (2004). Prefrontal cortical projections to the rat dorsal raphe nucleus: ultrastructural features and associations with serotonin and gamma-aminobutyric acid neurons. J Comp Neurol 468: 518–529.
Karasawa J, Shimazaki T, Kawashima N, Chaki S (2005). AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042: 92–98.
Kawashima N, Karasawa J, Shimazaki T, Chaki S, Okuyama S, Yasuhara A et al (2005). Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neurosci Lett 378: 131–134.
Koike H, Iijima M, Chaki S (2011a). Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology 61: 1419–1423.
Koike H, Iijima M, Chaki S (2011b). Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res 224: 107–111.
Koike H, Iijima M, Chaki S (2013a). Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav 107: 20–23.
Koike H, Fukumoto K, Iijima M, Chaki S (2013b). Role of BDNF/TrkB signaling in antidepressant-like effects of a group II metabotropic glutamate receptor antagonist in animal models of depression. Behav Brain Res 238: 48–52.
Krystal JH, Sanacora G, Duman RS (2013). Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 73: 1133–1141.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959–964.
López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A (2007). Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology 32: 2087–2097.
López-Gil X, Jiménez-Sánchez L, Romón T, Campa L, Artigas F, Adell A (2012). Importance of inter-hemispheric prefrontal connection in the effects of non-competitive NMDA receptor antagonists. Int J Neuropsychopharmacol 15: 945–956.
Maeng S, Zarate CA Jr, Du J, Schloesser RJ, McCammon J, Chen G (2008). Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry 63: 349–352.
Martín-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G et al (2001). Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J Neurosci 21: 9856–9866.
Moghaddam B, Adams B, Verma A, Daly D (1997). Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 17: 2921–2927.
Nishitani N, Nagayasu K, Asaoka N, Yamashiro M, Shirakawa H, Nakagawa T et al (2014). Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int J Neuropsychopharmacol 17: 1321–1326.
Nowak K, Meyza K, Nikolaev E, Hunt MJ, Kasicki S (2012). Local blockade of NMDA receptors in the rat prefrontal cortex increases c-Fos expression in multiple subcortical regions. Acta Neurobiol Exp (Wars) 72: 207–218.
Pałucha-Poniewiera A, Wierońska JM, Brański P, Stachowicz K, Chaki S, Pilc A (2010). On the mechanism of the antidepressant-like action of group II mGlu receptor antagonist, MGS0039. Psychopharmacology (Berl) 212: 523–535.
Paxinos G, Franklin K (1997). The mouse brain in stereotaxic coordinates. Academic, San Diego.
Pollak Dorocic I, Fürth D, Xuan Y, Johansson Y, Pozzi L, Silberberg G et al (2014). A whole-brain atlas of inputs to serotonergic neurons of the dorsal and median raphe nuclei. Neuron 83: 663–678.
Sugimoto Y, Yamamoto M, Tagawa N, Kobayashi Y, Mitsui-Saitoh K, Hotta Y et al (2011). Differences between mice strains in response to paroxetine in the forced swimming test: involvement of serotonergic or noradrenergic systems. Eur J Pharmacol 672: 121–125.
Varga V, Kocsis B, Sharp T (2003). Electrophysiological evidence for convergence of inputs from the medial prefrontal cortex and lateral habenula on single neurons in the dorsal raphe nucleus. Eur J Neurosci 17: 280–286.
Wang QP, Ochiai H, Nakai Y (1992). GABAergic innervation of serotonergic neurons in the dorsal raphe nucleus of the rat studied by electron microscopy double immunostaining. Brain Res Bull 29: 943–948.
Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY et al (2012). A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature 492: 428–432.
Weissbourd B, Ren J, DeLoach KE, Guenthner CJ, Miyamichi K, Luo L (2014). Presynaptic partners of dorsal raphe serotonergic and GABAergic neurons. Neuron 83: 645–662.
Yamanaka H, Yokoyama C, Mizuma H, Kurai S, Finnema SJ, Halldin C et al (2014). A possible mechanism of the nucleus accumbens and ventral pallidum 5-HT1B receptors underlying the antidepressant action of ketamine: a PET study with macaques. Transl Psychiatry 4: e342.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864.
Acknowledgements
We thank Drs Takao Yoshimizu and Daiji Kambe for technical advice for immunohistochemical studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Fukumoto, K., Iijima, M. & Chaki, S. The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacol 41, 1046–1056 (2016). https://doi.org/10.1038/npp.2015.233
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.233
This article is cited by
-
Are mGluR2/3 Inhibitors Potential Compounds for Novel Antidepressants?
Cellular and Molecular Neurobiology (2023)
-
The role of serotonin neurotransmission in rapid antidepressant actions
Psychopharmacology (2022)
-
Striatal Shati/Nat8l–BDNF pathways determine the sensitivity to social defeat stress in mice through epigenetic regulation
Neuropsychopharmacology (2021)
-
Frontal cortex genetic ablation of metabotropic glutamate receptor subtype 3 (mGlu3) impairs postsynaptic plasticity and modulates affective behaviors
Neuropsychopharmacology (2021)
-
Extrasynaptic CaMKIIα is involved in the antidepressant effects of ketamine by downregulating GluN2B receptors in an LPS-induced depression model
Journal of Neuroinflammation (2020)