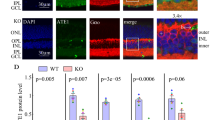
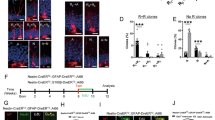
Abstract
GSK3β regulates some functions of the brain, but the mechanisms involved in the maintenance of GSK3β protein stability remain ambiguous. REGγ, an important proteasome activator for ubiquitin-independent protein degradation, has been shown to degrade certain intact proteins and is involved in the regulation of important biological processes. Here we demonstrate that REGγ promotes the degradation of GSK3β protein in vitro and in vivo. With increased GSK3β activity, REGγ knockout (REGγ−/−) mice exhibit late-onset sensorimotor gating and cognitive deficiencies including decreased working memory, hyperlocomotion, increased stereotype, defective prepulse inhibition (PPI), and disability in nest building, at the age of 8 months or older. Inhibition of GSK3β rescued the compromised PPI phenotypes and working memory deficiency in the knockout mice. Also, we found an age-dependent decrease in the trypsin-like proteasomal activity in REGγ−/− mice brains, which may be reflective of a lack of degradation of GSK3β. Collectively, our findings reveal a novel regulatory pathway in which the REGγ-proteasome controls the steady-state level of GSK3β protein. Dysfunction in this non-canonical proteasome degradation pathway may contribute to the sensorimotor gating deficiency and cognitive disorders in aging mice.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Agam G, Amar S, Kozlovsky N, Dean B, Scarr E, Belmaker R (2006). The possible role of the regulation of GSK-beta in schizophrenia and its treatment. Biol Psychiatry 59: 104S–104S.
Altar C, Jurata L, Charles V, Lemire A, Liu P, Bukhman Y et al (2005). Deficient hippocampal neuron expression of proteasome, ubiquitin, and mitochondrial genes in multiple schizophrenia cohorts. Biol Psychiatry 58: 85–96.
Barton L, Runnels H, Schell T, Cho Y, Gibbons R, Tevethia S et al (2004). Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol 172: 3948–3954.
Brewer GJ, Torricelli JR (2007). Isolation and culture of adult neurons and neurospheres. Nat Protoc 2: 1490–1498.
Cantor R, Kono N, Duvall J, Alvarez-Retuerto A, Stone J, Alarcon M et al (2005). Replication of autism linkage: fine-mapping peak at 17q21. Am J Hum Genet 76: 1050–1056.
Chan MH, Chiu PH, Lin CY, Chen HH (2012). Inhibition of glycogen synthase kinase-3 attenuates psychotomimetic effects of ketamine. Schizophr Res 136: 96–103.
Cline H (2003). Synaptic plasticity: importance of proteasome- mediated protein turnover. Curr Biol 13: R514–R516.
Datusalia AK, Sharma SS (2014). Amelioration of diabetes-induced cognitive deficits by GSK-3beta inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Mol Neurobiol 50: 390–405.
Doble B, Woodgett J (2003). GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116: 1175–1186.
Dong S, Jia C, Zhang S, Fan G, Li Y, Shan P et al (2013). The REG gamma proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab 18: 380–391.
Dubiel W, Pratt G, Ferrell K, Rechsteiner M (1992). Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem 267: 22369–22377.
Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM et al (1997). Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275: 661–665.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA (2004). Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36: 131–137.
Escamilla M, Hare E, Dassori A, Peralta J, Ontiveros A, Nicolini H et al (2009). A schizophrenia gene locus on chromosome 17q21 in a new set of families of Mexican and Central American ancestry: evidence from the NIMH genetics of schizophrenia in Latino populations study. Am J Psychiatry 166: 442–449.
Ewald H, Wikman F, Teruel B, Buttenschon H, Torralba M, Als T et al (2005). A genome-wide search for risk genes using homozygosity mapping and microarrays with 1,494 single-nucleotide polymorphisms in 22 eastern Cuban families with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet 133B: 25–30.
Freyberg Z, Ferrando SJ, Javitch JA (2010). Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167: 388–396.
Gray L, van den Buuse M, Scarr E, Dean B, Hannan AJ (2009). Clozapine reverses schizophrenia-related behaviours in the metabotropic glutamate receptor 5 knockout mouse: association with N-methyl-D-aspartic acid receptor up-regulation. Int J Neuropsychopharmacol 12: 45–60.
Jones C, Watson D, Fone K (2011). Animal models of schizophrenia. Br J Pharmacol 164: 1162–1194.
Kaake RM, Wang X, Huang L (2010). Profiling of protein interaction networks of protein complexes using affinity purification and quantitative mass spectrometry. Mol Cell Proteomics 9: 1650–1665.
Keller J, Gee J, Ding Q (2002). The proteasome in brain aging. Ageing Res Rev 1: 279–293.
Keller J, Hanni K, Markesbery W (2000). Impaired proteasome function in Alzheimer's disease. J Neurochem 75: 436–439.
Kobayashi T, Wang J, Al-Ahmadie H, Abate-Shen C (2013). ARF regulates the stability of p16 protein via REGγ-dependent proteasome degradation. Mol Cancer Res 11: 828–833.
Kozlovsky N, Belmaker R, Agam G (2001). Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr Res 52: 101–105.
Kozlovsky N, Belmaker R, Agam G (2002). GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol 12: 13–25.
Lewis C, Levinson D, Wise L, DeLisi L, Straub R, Hovatta I et al (2003). Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet 73: 34–48.
Li X, Amazit L, Long W, Lonard D, Monaco J, O'Malley B (2007). Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell 26: 831–842.
Li X, Lonard D, Jung S, Malovannaya A, Feng Q, Qin J et al (2006). The SRC-3/AIB1 coactivator is degraded in a ubiquitin- and ATP-independent manner by the REGgamma proteasome. Cell 124: 381–392.
Liu J, Yu G, Zhao Y, Zhao D, Wang Y, Wang L et al (2010). REGgamma modulates p53 activity by regulating its cellular localization. J Cell Sci 123 (Pt 23): 4076–4084.
Liu S, Lai L, Zuo Q, Dai F, Wu L, Wang Y et al (2014). PKA turnover by the REG gamma-proteasome modulates FoxO1 cellular activity and VEGF-induced angiogenesis. J Mol Cell Cardiol 72: 28–38.
Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V (2006). A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell 24: 627–633.
Lovestone S, Killick R, Di Forti M, Murray R (2007). Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci 30: 142–149.
Ma CP, Slaughter CA, DeMartino GN (1992). Identification, purification, and characterization of a protein activator (PA28) of the 20S proteasome (macropain). J Biol Chem 267: 10515–10523.
McNaught K, Olanow C, Halliwell B, Isacson O, Jenner P (2001). Failure of the ubiquitin-proteasome system in Parkinson's disease. Nat Rev Neurosci 2: 589–594.
Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y et al (1999). Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem 274: 38211–38215.
Rubio M, Wood K, Haroutunian V, Meador-Woodruff J (2013). Dysfunction of the ubiquitin proteasome and ubiquitin-like systems in schizophrenia. Neuropsychopharmacology 38: 1910–1920.
Seo H, Sonntag K, Kim W, Cattaneo E, Isacson O (2007). Proteasome activator enhances survival of Huntington's disease neuronal model cells. PLoS One 2: e238.
Speese S, Trotta N, Rodesch C, Aravamudan B, Broadie K (2003). The ubiquitin proteasome system acutely regulates presynaptic protein turnover and synaptic efficacy. Curr Biol 13: 899–910.
Stambolic V, Woodgett JR (1994). Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J 303 (Pt 3): 701–704.
Strucksberg KH, Tangavelou K, Schröder R, Clemen CS (2010). Proteasomal activity in skeletal muscle: a matter of assay design, muscle type, and age. Anal Biochem 399: 225–229.
Wang X, Huang L (2008). Identifying dynamic interactors of protein complexes by quantitative mass spectrometry. Mol Cell Proteomics 7: 46–57.
Watanabe Y, Khodosevich K, Monyer H (2014). Dendrite development regulated by the schizophrenia-associated gene FEZ1 involves the ubiquitin proteasome system. Cell Rep 7: 552–564.
WHO (2013) WHO Model List of Essential Medicines. World Health Organization, pp 7, 35.
Willi R, Harmeier A, Giovanoli S, Meyer U (2013). Altered GSK3 beta signaling in an infection-based mouse model of developmental neuropsychiatric disease. Neuropharmacology 73: 56–65.
Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT (1996). The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443–1454.
Yu G, Zhao Y, He J, Lonard DM, Mao CA, Wang G et al (2010). Comparative analysis of REG{gamma} expression in mouse and human tissues. J Mol Cell Biol 2: 192–198.
Zhang Z, Clawson A, Realini C, Jensen CC, Knowlton JR, Hill CP et al (1998). Identification of an activation region in the proteasome activator REGalpha. Proc Natl Acad Sci USA 95: 2807–2811.
Acknowledgements
We thank Professor Dongmin Yin and Zhiqi Xiong for their helpful suggestions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Lv, Y., Meng, B., Dong, H. et al. Upregulation of GSK3β Contributes to Brain Disorders in Elderly REGγ-knockout Mice. Neuropsychopharmacol 41, 1340–1349 (2016). https://doi.org/10.1038/npp.2015.285
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.285