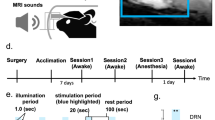
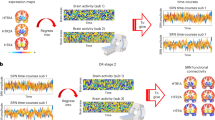
Abstract
Elucidating how the brain’s serotonergic network mediates diverse behavioral actions over both relatively short (minutes–hours) and long period of time (days–weeks) remains a major challenge for neuroscience. Our relative ignorance is largely due to the lack of technologies with robustness, reversibility, and spatio-temporal control. Recently, we have demonstrated that our chemogenetic approach (eg, Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)) provides a reliable and robust tool for controlling genetically defined neural populations. Here we show how short- and long-term activation of dorsal raphe nucleus (DRN) serotonergic neurons induces robust behavioral responses. We found that both short- and long-term activation of DRN serotonergic neurons induce antidepressant-like behavioral responses. However, only short-term activation induces anxiogenic-like behaviors. In parallel, these behavioral phenotypes were associated with a metabolic map of whole brain network activity via a recently developed non-invasive imaging technology DREAMM (DREADD Associated Metabolic Mapping). Our findings reveal a previously unappreciated brain network elicited by selective activation of DRN serotonin neurons and illuminate potential therapeutic and adverse effects of drugs targeting DRN neurons.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA et al (2009). Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63: 27–39.
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA 104: 5163–5168.
Berger M, Gray JA, Roth BL (2009). The expanded biology of serotonin. Annu Rev Med 60: 355–366.
Carter ME, Soden ME, Zweifel LS, Palmiter RD (2014). Genetic identification of a neural circuit that suppresses appetite. Nature 503: 111–114.
Chua P, Krams M, Toni I, Passingham R, Dolan R (1999). A functional anatomy of anticipatory anxiety. Neuroimage 9: 563–571.
Ciarleglio CM, Resuehr HE, McMahon DG (2011). Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 197: 8–16.
David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I et al (2009). Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron 62: 479–493.
Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ et al (2013). A Galphas DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38: 854–862.
Faure C, Mnie-Filali O, Haddjeri N (2006). Long-term adaptive changes induced by serotonergic antidepressant drugs. Expert Rev Neurother 6: 235–245.
Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS et al (2015). A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 18: 863–871.
Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N et al (2007). Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27: 9817–9823.
Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH (2008). A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA 105: 20575–20582.
Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF (1996). Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 54: 129–141.
Halberstadt AL, Balaban CD (2008). Selective anterograde tracing of nonserotonergic projections from dorsal raphe nucleus to the basal forebrain and extended amygdala. J Chem Neuroanat 35: 317–325.
Jacobs BL, Azmitia EC (1992). Structure and function of the brain serotonin system. Physiol Rev 72: 165–229.
Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J (2013). Chronic activation of a designer G(q)-coupled receptor improves beta cell function. J Clin Invest 123: 1750–1762.
Javanmard M, Shlik J, Kennedy SH, Vaccarino FJ, Houle S, Bradwejn J (1999). Neuroanatomic correlates of CCK-4-induced panic attacks in healthy humans: a comparison of two time points. Biol Psychiatry 45: 872–882.
Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S et al (2001). Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 158: 899–905.
Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS et al (2011). Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428.
Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS et al (2014). An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242.
Kroenke K, West SL, Swindle R, Gilsenan A, Eckert GJ, Dolor R et al (2001). Similar effectiveness of paroxetine, fluoxetine, and sertraline in primary care: a randomized trial. JAMA 286: 2947–2955.
Lam RW (2012). Onset, time course and trajectories of improvement with antidepressants. Eur Neuropsychopharmacol 22 (Suppl 3): S492–S498.
Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ et al (2008). Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann NY Acad Sci 1148: 86–94.
Luyten L, Casteels C, Vansteenwegen D, van Kuyck K, Koole M, Van Laere K et al (2012). Micro-positron emission tomography imaging of rat brain metabolism during expression of contextual conditioning. J Neurosci 32: 254–263.
Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL (2014). Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology 89: 157–167.
Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S et al (2000). Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 48: 830–843.
Michaelides M, Anderson SA, Ananth M, Smirnov D, Thanos PK, Neumaier JF et al (2013). Whole-brain circuit dissection in free-moving animals reveals cell-specific mesocorticolimbic networks. J Clin Invest 123: 5342–5350.
Michaelides M, Hurd YL (2015). DREAMM: a biobehavioral imaging methodology for dynamic in vivo whole-brain mapping of cell type-specific functional networks. Neuropsychopharmacology 40: 239–240.
Michaelides M, Pascau J, Gispert JD, Delis F, Grandy DK, Wang GJ et al (2010). Dopamine D4 receptors modulate brain metabolic activity in the prefrontal cortex and cerebellum at rest and in response to methylphenidate. Eur J Neurosci 32: 668–676.
Monti JM (2008) Serotonin and Sleep: Molecular, Functional and Clinical Aspects. Birkhäuser: Basel, Switzerland; Boston MA, USA.
Quitkin FM, Rabkin JG, Ross D, McGrath PJ (1984). Duration of antidepressant drug treatment. What is an adequate trial? Arch Gen Psychiatry 41: 238–245.
Rogan SC, Roth BL (2011). Remote control of neuronal signaling. Pharmacol Rev 63: 291–315.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al (2003). Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809.
Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW et al (2010). Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry 67: e9–e11.
Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z et al (2004). Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 22: 409–418.
Shumake J, Colorado RA, Barrett DW, Gonzalez-Lima F (2010). Metabolic mapping of the effects of the antidepressant fluoxetine on the brains of congenitally helpless rats. Brain Res 1343: 218–225.
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459: 698–702.
Stamford JA, Davidson C, McLaughlin DP, Hopwood SE (2000). Control of dorsal raphe 5-HT function by multiple 5-HT(1) autoreceptors: parallel purposes or pointless plurality? Trends Neurosci 23: 459–465.
Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH (2009). Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage 44: 975–981.
Sung KK, Jang DP, Lee S, Kim M, Lee SY, Kim YB et al (2009). Neural responses in rat brain during acute immobilization stress: a [F-18]FDG micro PET imaging study. Neuroimage 44: 1074–1080.
Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ et al (1999). Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry 46: 473–483.
Vertes RP (1991). A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol 313: 643–668.
Waselus M, Valentino RJ, Van Bockstaele EJ (2012). Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat 41: 266–280.
Willner P (1990). Animal models of depression: an overview. Pharmacol Ther 45: 425–455.
Wong KP, Sha W, Zhang X, Huang SC (2011). Effects of administration route, dietary condition, and blood glucose level on kinetics and uptake of 18F-FDG in mice. J Nucl Med 52: 800–807.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Urban, D., Zhu, H., Marcinkiewcz, C. et al. Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacol 41, 1404–1415 (2016). https://doi.org/10.1038/npp.2015.293
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.293
This article is cited by
-
Human tau-overexpressing mice recapitulate brainstem involvement and neuropsychiatric features of early Alzheimer’s disease
Acta Neuropathologica Communications (2023)
-
Organic cation transporter 2 contributes to SSRI antidepressant efficacy by controlling tryptophan availability in the brain
Translational Psychiatry (2023)
-
Chemogenetics for cell-type-specific modulation of signalling and neuronal activity
Nature Reviews Methods Primers (2023)
-
Repeated chemogenetic activation of dopaminergic neurons induces reversible changes in baseline and amphetamine-induced behaviors
Psychopharmacology (2023)
-
Dorsal striatal dopamine induces fronto-cortical hypoactivity and attenuates anxiety and compulsive behaviors in rats
Neuropsychopharmacology (2022)