Abstract
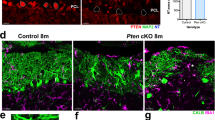
Autism spectrum disorders (ASDs) are neurodevelopmental disorders characterized by impaired social interaction, isolated areas of interest, and insistence on sameness. Mutations in Phosphatase and tensin homolog missing on chromosome 10 (PTEN) have been reported in individuals with ASDs. Recent evidence highlights a crucial role of the cerebellum in the etiopathogenesis of ASDs. In the present study we analyzed the specific contribution of cerebellar Purkinje cell (PC) PTEN loss to these disorders. Using the Cre-loxP recombination system, we generated conditional knockout mice in which PTEN inactivation was induced specifically in PCs. We investigated PC morphology and physiology as well as sociability, repetitive behavior, motor learning, and cognitive inflexibility of adult PC PTEN-mutant mice. Loss of PTEN in PCs results in autistic-like traits, including impaired sociability, repetitive behavior and deficits in motor learning. Mutant PCs appear hypertrophic and show structural abnormalities in dendrites and axons, decreased excitability, disrupted parallel fiber and climbing fiber synapses and late-onset cell death. Our results unveil new roles of PTEN in PC function and provide the first evidence of a link between the loss of PTEN in PCs and the genesis of ASD-like traits.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barski JJ, Dethleffsen K, Meyer M (2000). Cre recombinase expression in cerebellar Purkinje cells. Genesis 28: 93–98.
Baudouin SJ, Gaudias J, Gerharz S, Hatstatt L, Zhou K, Punnakkal P et al (2012). Shared synaptic pathophysiology in syndromic and nonsyndromic rodent models of autism. Science 338: 128–132.
Bosman LW, Konnerth A (2009). Activity-dependent plasticity of developing climbing fiber-Purkinje cell synapses. Neuroscience 162: 612–623.
Clipperton-Allen AE, Page DT (2014). Pten haploinsufficient mice show broad brain overgrowth but selective impairments in autism-relevant behavioral tests. Hum Mol Genet 23: 3490–3505.
Clipperton-Allen AE, Page DT (2015). Decreased aggression and increased repetitive behavior in Pten haploinsufficient mice. Genes Brain Behav 14: 145–157.
Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD et al (2001). Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57: 245–254.
Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ et al (2012). Consensus paper: pathological role of the cerebellum in autism. Cerebellum 11: 777–807.
Hashimoto K, Kano M (1998). Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. J Physiol 506: 391–405.
Jaworski J, Spangler S, Seeburg DP, Hoogenraad CC, Sheng M (2005). Control of dendritic arborization by the phosphoinositide-3′-kinase-Akt-mammalian target of rapamycin pathway. J Neurosci 25: 11300–11312.
Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W et al (2006). Pten regulates neuronal arborization and social interaction in mice. Neuron 50: 377–388.
Lai MC, Lombardo MV, Baron-Cohen S (2014). Autism. Lancet 383: 896–910.
Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al (1997). PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947.
Liu Y, Lee JW, Ackerman SL (2015). Mutations in the microtubule-associated protein 1A (Map1a) gene cause purkinje cell degeneration. J Neurosci 35: 4587–4598.
Lotta LT, Conrad K, Cory-Slechta D, Schor NF (2014). Cerebellar Purkinje cell p75 neurotrophin receptor and autistic behavior. Transl Psychiatry 4: e416.
Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL (2011). Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci 31: 4345–4354.
Marino S, Krimpenfort P, Leung C, van der Korput HA, Trapman J, Camenisch I et al (2002). PTEN is essential for cell migration but not for fate determination and tumourigenesis in the cerebellum. Development 129: 3513–3522.
Marko MK, Crocetti D, Hulst T, Donchin O, Shadmehr R, Mostofsky SH (2015). Behavioural and neural basis of anomalous motor learning in children with autism. Brain 138: 784–797.
Martin LA, Goldowitz D, Mittleman G (2010). Repetitive behavior and increased activity in mice with Purkinje cell loss: a model for understanding the role of cerebellar pathology in autism. Eur J Neurosci 31: 544–555.
McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF et al (2010). Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res 3: 137–141.
Mittleman G, Goldowitz D, Heck DH, Blaha CD (2008). Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse 62: 544–550.
Montarolo PG, Palestini M, Strata P (1982). The inhibitory effect of the olivocerebellar input on the cerebellar Purkinje cells in the rat. J Physiol 332: 187–202.
Muehlmann AM, Lewis MH (2012). Abnormal repetitive behaviours: shared phenomenology and pathophysiology. J Intellect Disabil Res 56: 427–440.
Muller RA (2007). The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev 13: 85–95.
Nayeem N, Kerr F, Naumann H, Linehan J, Lovestone S, Brandner S (2007). Hyperphosphorylation of tau and neurofilaments and activation of CDK5 and ERK1/2 in PTEN-deficient cerebella. Mol Cell Neurosci 34: 400–408.
Oberdick J, Smeyne RJ, Mann JR, Zackson S, Morgan JI (1990). A promoter that drives transgene expression in cerebellar Purkinje and retinal bipolar neurons. Science 248: 223–226.
Palmen SJ, van Engeland H, Hof PR, Schmitz C (2004). Neuropathological findings in autism. Brain 127: 2572–2583.
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B et al (2008). Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322: 963–966.
Pasciuto E, Borrie SC, Kanellopoulos AK, Santos AR, Cappuyns E, D'Andrea L et al (2015). Autism spectrum disorders: translating human deficits into mouse behavior. Neurobiol Learn Mem 124: 71–87.
Porras-García ME, Ruiz R, Pérez-Villegas EM, Armengol JÁ (2013). Motor learning of mice lacking cerebellar Purkinje cells. Front Neuroanat 7: 4.
Reeber SL, Otis TS, Sillitoe RV (2013). New roles for the cerebellum in health and disease. Front Syst Neurosci 7: 83.
Reith RM, McKenna J, Wu H, Hashmi SS, Cho SH, Dash PK et al (2013). Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol Dis 51: 93–103.
Reith RM, Way S, McKenna J 3rd, Haines K, Gambello MJ (2011). Loss of the tuberous sclerosis complex protein tuberin causes Purkinje cell degeneration. Neurobiol Dis 43: 113–122.
Rogers TD, Dickson PE, Heck DH, Goldowitz D, Mittleman G, Blaha CD (2011). Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse 65: 1204–1212.
Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS (2010). Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res 208: 178–188.
Safo PK, Cravatt BF, Regehr WG (2006). Retrograde endocannabinoid signaling in the cerebellar cortex. Cerebellum 5: 134–145.
Schmahmann JD (1998). Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 2: 362–371.
Skefos J, Cummings C, Enzer K, Holiday J, Weed K, Levy E et al (2014). Regional alterations in purkinje cell density in patients with autism. PLoS One 9: e81255.
Song MS, Salmena L, Pandolfi PP (2012). The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol 13: 283–296.
Strick PL, Dum RP, Fiez JA (2009). Cerebellum and nonmotor function. Annu Rev Neurosci 32: 413–434.
Takeuchi K, Gertner MJ, Zhou J, Parada LF, Bennett MV, Zukin RS (2013). Dysregulation of synaptic plasticity precedes appearance of morphological defects in a Pten conditional knockout mouse model of autism. Proc Natl Acad Sci USA 110: 4738–4743.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM et al (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488: 647–651.
Wang SS, Kloth AD, Badura A (2014). The cerebellum, sensitive periods, and autism. Neuron 83: 518–532.
Wegiel J, Flory M, Kuchna I, Nowicki K, Ma SY, Imaki H et al (2014). Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol Commun 2: 141.
Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ (2008). Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum 7: 406–416.
Won H, Lee HR, Gee HY, Mah W, Kim JI, Lee J et al (2012). Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature 486: 261–265.
Acknowledgements
We thank Marco Sassoè-Pognetto (University of Turin) for the kind gift of L7/CRE transgenic mice, Angela Longo (University of Turin) for her help with behavioral tests and Annalisa Buffo (University of Turin) for helpful discussion and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Cupolillo, D., Hoxha, E., Faralli, A. et al. Autistic-Like Traits and Cerebellar Dysfunction in Purkinje Cell PTEN Knock-Out Mice. Neuropsychopharmacol 41, 1457–1466 (2016). https://doi.org/10.1038/npp.2015.339
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.339
This article is cited by
-
Social memory deficit caused by dysregulation of the cerebellar vermis
Nature Communications (2023)
-
Cerebellum Lecture: the Cerebellar Nuclei—Core of the Cerebellum
The Cerebellum (2023)
-
Behavioural and psychological features of PTEN mutations: a systematic review of the literature and meta-analysis of the prevalence of autism spectrum disorder characteristics
Journal of Neurodevelopmental Disorders (2022)
-
Shank2/3 double knockout-based screening of cortical subregions links the retrosplenial area to the loss of social memory in autism spectrum disorders
Molecular Psychiatry (2022)
-
Conditional Pten knockout in parvalbumin- or somatostatin-positive neurons sufficiently leads to autism-related behavioral phenotypes
Molecular Brain (2021)