Abstract
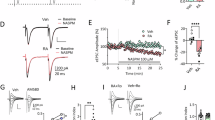
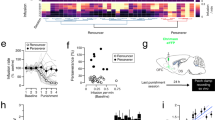
Addiction is a behavioral disease, of which core components can be modeled in rodents. Much evidence implicates drug-evoked synaptic plasticity in cocaine-evoked locomotor sensitization, cue-induced cocaine seeking, and incubation of cocaine craving. However, the type of plasticity evoked by different modalities of cocaine administration (eg contingent vs non-contingent) and its role in reshaping circuit function remains largely elusive. Here we exposed mice to various regimens of cocaine and recorded excitatory transmission onto identified medium-sized spiny neurons (MSN, expressing fluorescent proteins under the control of either D1R or D2R dopamine receptor promotor) in the nucleus accumbens at time points when behavioral adaptations are observed. In D1-MSN, we found the presence of GluA2-lacking α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) after single or chronic non-contingent exposure to cocaine as well as after cocaine self-administration (SA). We also report an increase in the AMPA/NMDA ratio (A/N) in D1-MSN, which was observed only after repeated passive injections associated with locomotor sensitization as well as in a condition of SA leading to seeking behavior. Remarkably, insertion of GluA2-lacking AMPARs was also detected in D2-MSN after SA of a high dose of cocaine but not regular dose (1.5 vs 0.75 mg/kg), which was the only condition where incubation of cocaine craving was observed in this study. Moreover, synapses containing GluA2-lacking AMPARs belonged to amygdala inputs in D2-MSN and to medial prefrontal cortex inputs in D1-MSN. Taken together this study allows for a refinement of a circuit model of addiction based on specific synaptic changes induced by cocaine.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ahmed SH, Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300.
Badiani A, Robinson TE (2004). Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol 15: 327–339.
Bellone C, Lüscher C, Mameli M (2008). Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci 65: 2913–2923.
Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF et al (2013). Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 16: 632–638.
Boudreau AC (2005). Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci 25: 9144–9151.
Boudreau AC, Reimers JM, Milovanovic M, Wolf ME (2007). Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci 27: 10621–10635.
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A (2012). Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76: 790–803.
Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN et al (2011). A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci 31: 8163–8174.
Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J et al (2002). Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22: 2977–2988.
Carelli RM, West EA (2014). Neuropharmacology. Neuropharmacology 76: 360–369.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L-J, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Creed M, Pascoli VJ, Lüscher C (2015). Addiction therapy. Refining deep brain stimulation to emulate optogenetic treatment of synaptic pathology. Science 347: 659–664.
Danjo T, Yoshimi K, Funabiki K, Yawata S, Nakanishi S (2014). Aversive behavior induced by optogenetic inactivation of ventral tegmental area dopamine neurons is mediated by dopamine D2 receptors in the nucleus accumbens. Proc Natl Acad Sci USA 111: 6455–6460.
Gawin FH, Morgan C, Kosten TR, Kleber HD (1989). Double-blind evaluation of the effect of acute amantadine on cocaine craving. Psychopharmacol 97: 402–403.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Nature 412: 141–142.
Halbout B, Bernardi RE, Hansson AC, Spanagel R (2014). Incubation of cocaine seeking following brief cocaine experience in mice is enhanced by mGluR1 blockade. J Neurosci 34: 1781–1790.
Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G et al (2009). In vivo cocaine experience generates silent synapses. Neuron 63: 40–47.
Jonas P, Burnashev N (1995). Molecular mechanisms controlling calcium entry through AMPA-type glutamate receptor channels. Neuron 15: 987–990.
Kourrich S, Rothwell PE, Klug JR, Thomas MJ (2007). Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci 27: 7921–7928.
Lee BR, Dong Y (2011). Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond. Neuropharmacology 61: 1060–1069.
Lee BR, Ma Y-Y, Huang YH, Wang X, Otaka M, Ishikawa M et al (2013). Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 2013 16: 1644–1651.
Liu SJ, Zukin RS (2007). Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30: 126–134.
Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT et al (2014). Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 17: 73–80.
Lüscher C (2013). Drug-evoked synaptic plasticity causing addictive Behavior. J Neurosci 33: 17641–17646.
Lüscher C, Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663.
Ma Y-Y, Lee BR, Wang X, Guo C, Liu L, Cui R et al (2014). Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83: 1453–1467.
Mameli M, Bellone C, Brown MTC, Lüscher C (2011). Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci 14: 414–416.
Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R et al (2009). Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12: 1036–1041.
Mccutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M (2011). Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci 31: 5737–5743.
Nelson AB, Hang GB, Grueter BA, Pascoli V, Lüscher C, Malenka RC et al (2012). A comparison of striatal-dependent behaviors in wild-type and hemizygous Drd1a and Drd2 BAC transgenic mice. J Neurosci 32: 9119–9123.
Nestler EJ (2013). Cellular basis of memory for addiction. Dialogues Clin Neurosci 15: 431–443.
Ortinski PI, Vassoler FM, Carlson GC, Pierce RC (2012). Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology 37: 1671–1682.
Pascoli V, Terrier J, Espallergues J, Valjent E, O’Connor EC, Lüscher C (2014). Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509: 459–464.
Pascoli V, Turiault M, Lüscher C (2011). Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 481: 71–75.
Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y (2011). Neurobiology of the incubation of drug craving. Trends Neurosci 34: 411–420.
Pierce RC, Bell K, Duffy P, Kalivas PW (1996). Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci 16: 1550–1560.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 18: 247–291.
Steketee JD, Kalivas PW (2011). Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63: 348–365.
Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault J-A (2010). Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology 35: 401–415.
Valjent E, Corvol J-C, Trzaskos J, Girault J-A, Hervé D (2006). Valjent_2006_BMC Neuroscience ERK locomotion. BMC Neurosci 7: 20.
Verdoorn TA, Burnashev N, Monyer H, Seeburg PH, Sakmann B (1991). Structural determinants of ion flow through recombinant glutamate receptor channels. Science 252: 1715–1718.
Wolf ME, Ferrario CR (2010). AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev 35: 185–211.
Xi Z-X, Li X, Li J, Peng X-Q, Song R, Gaál J et al (2012). Blockade of dopamine D3 receptors in the nucleus accumbens and central amygdala inhibits incubation of cocaine craving in rats. Addict Biol 18: 665–677.
Acknowledgements
We thank the members of the Lüscher lab for many discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Terrier, J., Lüscher, C. & Pascoli, V. Cell-Type Specific Insertion of GluA2-Lacking AMPARs with Cocaine Exposure Leading to Sensitization, Cue-Induced Seeking, and Incubation of Craving. Neuropsychopharmacol 41, 1779–1789 (2016). https://doi.org/10.1038/npp.2015.345
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.345
This article is cited by
-
Membrane excitability of nucleus accumbens neurons gates the incubation of cocaine craving
Neuropsychopharmacology (2023)
-
Whole-brain tracking of cocaine and sugar rewards processing
Translational Psychiatry (2023)
-
Sleep-mediated regulation of reward circuits: implications in substance use disorders
Neuropsychopharmacology (2023)
-
Persistent increase of accumbens cocaine ensemble excitability induced by IRK downregulation after withdrawal mediates the incubation of cocaine craving
Molecular Psychiatry (2023)
-
Cocaine restricts nucleus accumbens feedforward drive through a monoamine-independent mechanism
Neuropsychopharmacology (2022)