Abstract
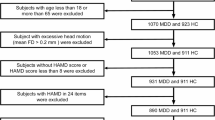
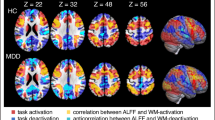
Major depressive disorder (MDD) is characterized by abnormal resting-state functional connectivity (RSFC), especially in medial prefrontal cortical (MPFC) regions of the default network. However, prior research in MDD has not examined dynamic changes in functional connectivity as networks form, interact, and dissolve over time. We compared unmedicated individuals with MDD (n=100) to control participants (n=109) on dynamic RSFC (operationalized as SD in RSFC over a series of sliding windows) of an MPFC seed region during a resting-state functional magnetic resonance imaging scan. Among participants with MDD, we also investigated the relationship between symptom severity and RSFC. Secondary analyses probed the association between dynamic RSFC and rumination. Results showed that individuals with MDD were characterized by decreased dynamic (less variable) RSFC between MPFC and regions of parahippocampal gyrus within the default network, a pattern related to sustained positive connectivity between these regions across sliding windows. In contrast, the MDD group exhibited increased dynamic (more variable) RSFC between MPFC and regions of insula, and higher severity of depression was related to increased dynamic RSFC between MPFC and dorsolateral prefrontal cortex. These patterns of highly variable RSFC were related to greater frequency of strong positive and negative correlations in activity across sliding windows. Secondary analyses indicated that increased dynamic RSFC between MPFC and insula was related to higher levels of recent rumination. These findings provide initial evidence that depression, and ruminative thinking in depression, are related to abnormal patterns of fluctuating communication among brain systems involved in regulating attention and self-referential thinking.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014). Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24: 663–676.
Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron 65: 550–562.
Andrews-Hanna JR, Smallwood J, Spreng RN (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann NY Acad Sci 1316: 29–52.
Banich MT, Milham MP, Atchley RA, Cohen NJ, Webb A, Wszalek T et al (2000). Prefrontal regions play a predominant role in imposing an attentional ‘set’: evidence from fMIRI. Cogn Brain Res 10: 1–9.
Beck AT, Steer RA, Ball R, Ranieri WF (1996). Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess 67: 588–597.
Behzadi Y, Restom K, Liau J, Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37: 90–101.
Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34: 537–541.
Bollen KA, Jackman RW (1985). Regression diagnostics: an expository treatment of outliers and influential cases. Sociol Methods Res 13: 510–542.
Bray S, Arnold A, Levy RM, Iaria G (2015). Spatial and temporal functional connectivity changes between resting and attentive states. Hum Brain Mapp 36: 549–565.
Buckner RL, Krienen FM, Yeo BTT (2013). Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16: 832–837.
Chang C, Glover GH (2010). Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50: 81–98.
Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M (2013a). Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 68: 93–104.
Chang LJ, Yarkoni T, Khaw MW, Sanfey AG (2013b). Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 23: 739–749.
Cook RD (1977). Detection of influential observation in linear regression. Technometrics 19: 15–18.
Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH et al (2001). Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol 22: 1326–1333.
Craig AD (2009). How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70.
Cribben I, Haraldsdottir R, Atlas LY, Wager TD, Lindquist MA (2012). Dynamic connectivity regression: determining state-related changes in brain connectivity. Neuroimage 61: 907–920.
Crowther A, Smoski MJ, Minkel J, Moore T, Gibbs D, Petty C et al (2015). Resting-state connectivity predictors of response to psychotherapy in major depressive disorder. Neuropsychopharmacology 40: 1659–1673.
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL et al (2013). Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med 10: 12.
Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD (1996). Detecting activations in PET and fMRI: levels of inference and power. Neuroimage 4: 223–235.
Gasquoine PG (2014). Contributions of the insula to cognition and emotion. Neuropsychol Rev 24: 77–87.
Gonzalez-Castillo J, Handwerker DA, Robinson ME, Hoy CW, Buchanan LC, Saad ZS et al (2014). The spatial structure of resting state connectivity stability on the scale of minutes. Front Neurosci 8: 19.
Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M et al (2013a). Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80: 360–378.
Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS (2013b). Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Hum Brain Mapp 34: 2154–2177.
Jones DT, Vemuri P, Murphy MC, Gunter JL, Senjem ML, Machulda MM et al (2012). Non-stationarity in the “resting brain’s” modular architecture. PLoS One 7: 15.
Kaiser RH, Andrews-Hanna JR, Spielberg JM, Warren SL, Sutton BP, Miller GA et al (2014). Distracted and down: neural mechanisms of affective interference in subclinical depression. Soc Cogn Affect Neurosci 10: 654–663.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72: 603–611.
Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR (2007). The Behavioral Activation for Depression Scale (BADS): psychometric properties and factor structure. J Psychopathol Behav Assess 29: 191–202.
Kessler RC, Petukhova M, Sampson NA, Zaslavsky AM, Wittchen HU (2012). Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res 21: 169–184.
Leonardi N, Van De Ville D (2015). On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage 104: 430–436.
Ma S, Calhoun VD, Phlypo R, Adali T (2014). Dynamic changes of spatial functional network connectivity in individuals and schizophrenia patients using independent vector analysis. Neuroimage 90: 196–206.
MacDonald AW, Cohen JD, Stenger VA, Carter CS (2000). Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838.
Marchetti I, Koster EHW, Sonuga-Barke EJS, De Raedt R (2012). The default mode network and recurrent depression: a neurobiological model of cognitive risk factors. Neuropsychol Rev 22: 229–251.
Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA et al (1999). Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682.
Menon V, Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667.
Meunier D, Lambiotte R, Fornito A, Ersche K, Bullmore E (2009). Hierarchical modularity in human brain functional networks. Front Hum Neurosci 3: 37.
Nelson SM, Dosenbach NUF, Cohen AL, Wheeler ME, Schlaggar BL, Petersen SE (2010). Role of the anterior insula in task-level control and focal attention. Brain Struct Funct 214: 669–680.
Rashid B, Damaraju E, Pearlson GD, Calhoun VD (2014). Dynamic connectivity states estimated from resting fMRI identify differences among schizophrenia, bipolar disorder, and healthy control subjects. Front Hum Neurosci 8: 13.
Shen K, Hutchison RM, Bezgin G, Everling S, McIntosh AR (2015). Network structure shapes spontaneous functional connectivity dynamics. J Neurosci 35: 5579–5588.
Sridharan D, Levitin DJ, Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 105: 12569–12574.
Thompson GJ, Magnuson ME, Merritt MD, Schwarb H, Pan WJ, McKinley A et al (2013). Short-time windows of correlation between large-scale functional brain networks predict vigilance intraindividually and interindividually. Hum Brain Mapp 34: 3280–3298.
van Tol MJ, Li M, Metzger CD, Hailla N, Horn DI, Li W et al (2014). Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med 44: 2053–2065.
Vul E, Harris C, Winkielman P, Pashler H (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 4: 274–290.
Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology. In: NolenHoeksema S (ed). Annual Review of Clinical Psychology, Vol. 8. Annual Reviews: Palo Alto, pp 49.
Whitfield-Gabrieli S, Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2: 125–141.
Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165.
Zanto TP, Gazzaley A (2013). Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci 17: 602–603.
Acknowledgements
We thank Alfonso Nieto-Castanon for valuable discussion of analytic approach, and Min Su Kang for help in manuscript preparation. This work was partially supported by the Rappaport Mental Health Research Fellowship and National Institute of Mental Health (NIMH) grant F32 MH106262 awarded to RHK, NIMH grant R00 MH094438 awarded to DGD, NIMH grants R21 MH094781 and R21 MH094781 S1 awarded to MS and GD, and NIMH grants R01 MH068376 and R01 MH101521 awarded to DAP.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Kaiser, R., Whitfield-Gabrieli, S., Dillon, D. et al. Dynamic Resting-State Functional Connectivity in Major Depression. Neuropsychopharmacol 41, 1822–1830 (2016). https://doi.org/10.1038/npp.2015.352
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.352
This article is cited by
-
Aberrant resting-state co-activation network dynamics in major depressive disorder
Translational Psychiatry (2024)
-
Association of executive function with suicidality based on resting-state functional connectivity in young adults with subthreshold depression
Scientific Reports (2023)
-
Functional connectivity uniqueness and variability? Linkages with cognitive and psychiatric problems in children
Nature Mental Health (2023)
-
A dorsomedial prefrontal cortex-based dynamic functional connectivity model of rumination
Nature Communications (2023)
-
High and low worriers do not differ in unstimulated resting-state brain connectivity
Scientific Reports (2023)