Abstract
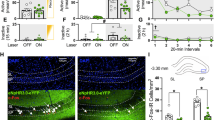
Cocaine-associated environmental cues sustain relapse vulnerability by reactivating long-lasting memories of cocaine reward. During periods of abstinence, responding to cocaine cues can time-dependently intensify a phenomenon referred to as ‘incubation of cocaine craving’. Here, we investigated the role of the extracellular matrix protein brevican in recent (1 day after training) and remote (3 weeks after training) expression of cocaine conditioned place preference (CPP). Wild-type and Brevican heterozygous knock-out mice, which express brevican at ~50% of wild-type levels, received three cocaine–context pairings using a relatively low dose of cocaine (5 mg/kg). In a drug-free CPP test, heterozygous mice showed enhanced preference for the cocaine-associated context at the remote time point compared with the recent time point. This progressive increase was not observed in wild-type mice and it did not generalize to contextual-fear memory. Virally mediated overexpression of brevican levels in the hippocampus, but not medial prefrontal cortex, of heterozygous mice prevented the progressive increase in cocaine CPP, but only when overexpression was induced before conditioning. Post-conditioning overexpression of brevican did not affect remote cocaine CPP, suggesting that brevican limited the increase in remote CPP by altering neuro-adaptive mechanisms during cocaine conditioning. We provide causal evidence that hippocampal brevican levels control time-dependent enhancement of cocaine CPP during abstinence, pointing to a novel substrate that regulates incubation of responding to cocaine-associated cues.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aspberg A, Miura R, Bourdoulous S, Shimonaka M, Heinegard D, Schachner M et al (1997). The C-type lectin domains of lecticans, a family of aggregating chondroitin sulfate proteoglycans, bind tenascin-R by protein-protein interactions independent of carbohydrate moiety. Proc Natl Acad Sci USA 94: 10116–10121.
Brakebusch C, Seidenbecher CI, Asztely F, Rauch U, Matthies H, Meyer H et al (2002). Brevican-deficient mice display impaired hippocampal CA1 long-term potentiation but show no obvious deficits in learning and memory. Mol Cell Biol 22: 7417–7427.
Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW, Sorg BA (2007). Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem 14: 214–223.
Bruckner G, Hartig W, Kacza J, Seeger J, Welt K, Brauer K (1996). Extracellular matrix organization in various regions of rat brain grey matter. J Neurocytol 25: 333–346.
Carulli D, Rhodes KE, Brown DJ, Bonnert TP, Pollack SJ, Oliver K et al (2006). Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J Comp Neurol 494: 559–577.
Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y et al (2008). Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 454: 118–121.
Dityatev A, Bruckner G, Dityateva G, Grosche J, Kleene R, Schachner M (2007). Activity-dependent formation and functions of chondroitin sulfate-rich extracellular matrix of perineuronal nets. Dev Neurobiol 67: 570–588.
Dityatev A, Schachner M (2003). Extracellular matrix molecules and synaptic plasticity. Nat Rev Neurosci 4: 456–468.
Frankland PW, Bontempi B (2005). The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130.
Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ (2004). The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304: 881–883.
Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED (2009). Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12: 897–904.
Frischknecht R, Seidenbecher CI (2012). Brevican: a key proteoglycan in the perisynaptic extracellular matrix of the brain. Int J Biochem Cell Biol 44: 1051–1054.
Goshen I, Brodsky M, Prakash R, Wallace J, Gradinaru V, Ramakrishnan C et al (2011). Dynamics of retrieval strategies for remote memories. Cell 147: 678–689.
Grimm JW, Hope BT, Wise RA, Shaham Y (2001). Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature 412: 141–142.
Gundelfinger ED, Frischknecht R, Choquet D, Heine M (2010). Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci 31: 2156–2165.
Hagihara K, Miura R, Kosaki R, Berglund E, Ranscht B, Yamaguchi Y (1999). Immunohistochemical evidence for the brevican-tenascin-R interaction: colocalization in perineuronal nets suggests a physiological role for the interaction in the adult rat brain. J Comp Neurol 410: 256–264.
Kalivas PW, O’Brien C (2008). Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33: 166–180.
Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009). Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56 (Suppl 1): 177–185.
Li YQ, Li FQ, Wang XY, Wu P, Zhao M, Xu CM et al (2008). Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci 28: 13248–13257.
Loos M, Mueller T, Gouwenberg Y, Wijnands R, van der Loo RJ, Birchmeier C et al (2014). Neuregulin-3 in the Mouse Medial Prefrontal Cortex Regulates Impulsive Action. Biol Psychiatry. 76: 648–655.
Loos M, van der Sluis S, Bochdanovits Z, van Zutphen IJ, Pattij T, Stiedl O et al (2009). Activity and impulsive action are controlled by different genetic and environmental factors. Genes Brain Behav 8: 817–828.
Lu L, Grimm JW, Hope BT, Shaham Y (2004). Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47 (Suppl 1): 214–226.
Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005). Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8: 212–219.
Lubbers BR, Smit AB, Spijker S, van den Oever MC (2014a). Neural ECM in addiction, schizophrenia, and mood disorder. Prog Brain Res 214: 263–284.
Lubbers BR, van Mourik Y, Schetters D, Smit AB, De Vries TJ, Spijker S (2014b). Prefrontal gamma-aminobutyric acid type a receptor insertion controls cue-induced relapse to nicotine seeking. Biol Psychiatry 76: 750–758.
Luscher C, Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663.
Malinow R, Malenka RC (2002). AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126.
Maren S, Phan KL, Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14: 417–428.
Mash DC, ffrench-Mullen J, Adi N, Qin Y, Buck A, Pablo J (2007). Gene expression in human hippocampus from cocaine abusers identifies genes which regulate extracellular matrix remodeling. PLoS One 2: e1187.
Meyers RA, Zavala AR, Speer CM, Neisewander JL (2006). Dorsal hippocampus inhibition disrupts acquisition and expression, but not consolidation, of cocaine conditioned place preference. Behav Neurosci 120: 401–412.
Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R (2014). Engineering a memory with LTD and LTP. Nature 511: 348–352.
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002). Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298: 1248–1251.
Rajasethupathy P, Sankaran S, Marshel JH, Kim CK, Ferenczi E, Lee SY et al (2015). Projections from neocortex mediate top-down control of memory retrieval. Nature 526: 653–659.
Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB et al (2011). Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci 14: 1302–1308.
Robinson TE, Kolb B (2004). Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 47 (Suppl 1): 33–46.
Seidenbecher CI, Richter K, Rauch U, Fassler R, Garner CC, Gundelfinger ED (1995). Brevican, a chondroitin sulfate proteoglycan of rat brain, occurs as secreted and cell surface glycosylphosphatidylinositol-anchored isoforms. J Biol Chem 270: 27206–27212.
Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J et al (2015). Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci 35: 4190–4202.
Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA et al (2014). Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci 17: 1655–1657.
Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ (2014). Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron 84: 347–354.
Tzschentke TM, Schmidt WJ (1999). Functional heterogeneity of the rat medial prefrontal cortex: effects of discrete subarea-specific lesions on drug-induced conditioned place preference and behavioural sensitization. Eur J Neurosci 11: 4099–4109.
Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN et al (2008). Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci 11: 1053–1058.
Van den Oever MC, Lubbers BR, Goriounova NA, Li KW, Van der Schors RC, Loos M et al (2010). Extracellular matrix plasticity and GABAergic inhibition of prefrontal cortex pyramidal cells facilitates relapse to heroin seeking. Neuropsychopharmacology 35: 2120–2133.
Van den Oever MC, Rotaru DC, Heinsbroek JA, Gouwenberg Y, Deisseroth K, Stuber GD et al (2013). Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J Neurosci 33: 18225–18233.
Van den Oever MC, Spijker S, Smit AB (2012). The synaptic pathology of drug addiction. Adv Exp Med Biol 970: 469–491.
Vegh MJ, Heldring CM, Kamphuis W, Hijazi S, Timmerman AJ, Li KW et al (2014). Reducing hippocampal extracellular matrix reverses early memory deficits in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun 2: 76.
Viapiano MS, Matthews RT, Hockfield S (2003). A novel membrane-associated glycovariant of BEHAB/brevican is up-regulated during rat brain development and in a rat model of invasive glioma. J Biol Chem 278: 33239–33247.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Lubbers, B., Matos, M., Horn, A. et al. The Extracellular Matrix Protein Brevican Limits Time-Dependent Enhancement of Cocaine Conditioned Place Preference. Neuropsychopharmacol 41, 1907–1916 (2016). https://doi.org/10.1038/npp.2015.361
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.361
This article is cited by
-
Net gain and loss: influence of natural rewards and drugs of abuse on perineuronal nets
Neuropsychopharmacology (2023)
-
From stress to depression: development of extracellular matrix-dependent cognitive impairment following social stress
Scientific Reports (2020)
-
Memory strength gates the involvement of a CREB-dependent cortical fear engram in remote memory
Nature Communications (2019)
-
Engram-specific transcriptome profiling of contextual memory consolidation
Nature Communications (2019)
-
Müller glial microRNAs are required for the maintenance of glial homeostasis and retinal architecture
Nature Communications (2017)