Abstract
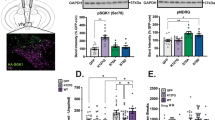
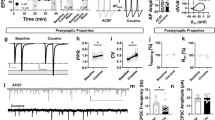
Cocaine addiction continues to be a significant public health problem for which there are currently no effective FDA-approved treatments. Thus, there is a clear need to identify and develop novel pharmacotherapies for cocaine addiction. Recent evidence indicates that activation of glucagon-like peptide-1 (GLP-1) receptors in the ventral tegmental area (VTA) reduces intake of highly palatable food. As the neural circuits and neurobiological mechanisms underlying drug taking overlap to some degree with those regulating food intake, these findings suggest that activation of central GLP-1 receptors may also attenuate cocaine taking. Here, we show that intra-VTA administration of the GLP-1 receptor agonist exendin-4 (0.05 μg) significantly reduced cocaine, but not sucrose, self-administration in rats. We also demonstrate that cocaine taking is associated with elevated plasma corticosterone levels and that systemic infusion of cocaine activates GLP-1-expressing neurons in the nucleus tractus solitarius (NTS), a hindbrain nucleus that projects monosynaptically to the VTA. To determine the potential mechanisms by which cocaine activates NTS GLP-1-expressing neurons, we microinjected corticosterone (0.5 μg) directly into the hindbrain fourth ventricle. Intraventricular corticosterone attenuated cocaine self-administration and this effect was blocked in animals pretreated with the GLP-1 receptor antagonist exendin-(9–39) (10 μg) in the VTA. Finally, AAV-shRNA-mediated knockdown of VTA GLP-1 receptors was sufficient to augment cocaine self-administration. Taken together, these findings indicate that increased activation of NTS GLP-1-expressing neurons by corticosterone may represent a homeostatic response to cocaine taking, thereby reducing the reinforcing efficacy of cocaine. Therefore, central GLP-1 receptors may represent a novel target for cocaine addiction pharmacotherapies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Alhadeff AL, Rupprecht LE, Hayes MR (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658.
Arnold JM, Roberts DC (1997). A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav 57: 441–447.
Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ (2011). Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31: 3904–3913.
Buffalari DM, Rinaman L (2014). Cocaine self-administration and extinction alter medullary noradrenergic and limbic forebrain cFos responses to acute, noncontingent cocaine injections in adult rats. Neuroscience 281C: 241–250.
Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH et al (2009). Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374: 39–47.
Deroche V, Marinelli M, Le Moal M, Piazza PV (1997). Glucocorticoids and behavioral effects of psychostimulants. II: cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther 281: 1401–1407.
Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012). The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820.
Egecioglu E, Engel JA, Jerlhag E (2013a). The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS One 8: e77284.
Egecioglu E, Engel JA, Jerlhag E (2013b). The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One 8: e69010.
Egecioglu E, Steensland P, Fredriksson I, Feltmann K, Engel JA, Jerlhag E (2013c). The glucagon-like peptide 1 analogue Exendin-4 attenuates alcohol mediated behaviors in rodents. Psychoneuroendocrinology 38: 1259–1270.
Engel JA, Jerlhag E (2014). Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs 28: 875–886.
Galici R, Pechnick RN, Poland RE, France CP (2000). Comparison of noncontingent versus contingent cocaine administration on plasma corticosterone levels in rats. Eur J Pharmacol 387: 59–62.
Goeders NE, Guerin GF (1996). Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology 64: 337–348.
Goeders NE, Guerin GF (2008). Effects of the combination of metyrapone and oxazepam on cocaine and food self-administration in rats. Pharmacol Biochem Behav 91: 181–189.
Grabus SD, Glowa JR, Riley AL (2004). Morphine- and cocaine-induced c-Fos levels in Lewis and Fischer rat strains. Brain Res 998: 20–28.
Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR et al (2013). Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 33: 11800–11810.
Graham DL, Erreger K, Galli A, Stanwood GD (2013). GLP-1 analog attenuates cocaine reward. Mol Psychiatry 18: 961–962.
Grill HJ, Hayes MR (2012). Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metabol 16: 296–309.
Gu G, Roland B, Tomaselli K, Dolman CS, Lowe C, Heilig JS (2013). Glucagon-like peptide-1 in the rat brain: distribution of expression and functional implication. J Comp Neurol 521: 2235–2261.
Guerin GF, Schmoutz CD, Goeders NE (2014). The extra-adrenal effects of metyrapone and oxazepam on ongoing cocaine self-administration. Brain Res 1575: 45–54.
Gutzwiller JP, Drewe J, Goke B, Schmidt H, Rohrer B, Lareida J et al (1999a). Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol 276: R1541–R1544.
Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D et al (1999b). Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 44: 81–86.
Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK (1986). Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res 16: 97–107.
Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD et al (2015). Septal glucagon-like peptide 1 receptor expression determines suppression of cocaine-induced behavior. Neuropsychopharmacology 40: 1969–1978.
Hayes MR, Bradley L, Grill HJ (2009). Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659.
Hayes MR, De Jonghe BC, Kanoski SE (2010). Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100: 503–510.
Hodos W (1961). Progressive ratio as a measure of reward strength. Science 134: 943–944.
Holland RA, Leonard JJ, Kensey NA, Hannikainen PA, De Jonghe BC (2014). Cisplatin induces neuronal activation and increases central AMPA and NMDA receptor subunit gene expression in mice. Physiol Behav 136: 79–85.
Holst JJ (2007). The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439.
Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR (2011). Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112.
Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR (2012). The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 62: 1916–1927.
Kenny PJ (2011a). Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci 12: 638–651.
Kenny PJ (2011b). Reward mechanisms in obesity: new insights and future directions. Neuron 69: 664–679.
Larsen PJ, Tang-Christensen M, Holst JJ, Orskov C (1997). Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 77: 257–270.
Lovshin JA, Drucker DJ (2009). Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269.
Mantsch JR, Schlussman SD, Ho A, Kreek MJ (2000). Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. J Pharmacol Exp Ther 294: 239–247.
Merchenthaler I, Lane M, Shughrue P (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280.
Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC et al (2013). The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 305: E1367–E1374.
Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ et al (2015). Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology 40: 372–385.
Narayanan NS, Guarnieri DJ, DiLeone RJ (2010). Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol 31: 104–112.
Paxinos G, Watson C (1997) The Rat Brain in Stereotaxic Coordinates. Academic Press: New York.
Pierce RC, Kumaresan V (2006). The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev 30: 215–238.
Richardson NR, Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66: 1–11.
Rinaman L (2010). Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34.
Ruttimann EB, Arnold M, Hillebrand JJ, Geary N, Langhans W (2009). Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology 150: 1174–1181.
Schmidt HD, Anderson SM, Famous KR, Kumaresan V, Pierce RC (2005). Anatomy and pharmacology of cocaine priming-induced reinstatement of drug seeking. Eur J Pharmacol 526: 65–76.
Schmidt HD, Famous KR, Pierce RC (2009). The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci 30: 1358–1369.
Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS et al (2014). The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 124: 4473–4488.
Shirazi RH, Dickson SL, Skibicka KP (2013). Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One 8: e61965.
Shukla AP, Buniak WI, Aronne LJ (2015). Treatment of obesity in 2015. J Cardiopulm Rehabil Prev 35: 81–92.
Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ (2014). Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest 124: 2456–2463.
Skibicka KP (2013). The central GLP-1: implications for food and drug reward. Front Neurosci 7: 181.
Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K et al (1996). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379: 69–72.
Williams KW, Elmquist JK (2012). From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15: 1350–1355.
Zahm DS, Becker ML, Freiman AJ, Strauch S, Degarmo B, Geisler S et al (2010). Fos after single and repeated self-administration of cocaine and saline in the rat: emphasis on the basal forebrain and recalibration of expression. Neuropsychopharmacology 35: 445–463.
Zhang R, Packard BA, Tauchi M, D'Alessio DA, Herman JP (2009). Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci USA 106: 5913–5918.
Acknowledgements
This work was supported by the following grants from the National Institutes of Health: K01 DA030445 and R01 DA037897 (to HDS), F32 DK097954 and K01 DK103804 (to EGM-B), T32 DA28874 (to LAG and MEW), T32 MH014654 (to DJR), and R01 DK096139 (MRH). HDS and KYI were partially supported by a Vagelos Undergraduate Research Grant from the Center for Undergraduate Research & Fellowships at the University of Pennsylvania. We also thank Adrian Arreola, Christopher Turner, Nicole Hernandez, and Lauren McGrath for their technical assistance as well as Dr Kendra Bence for use of equipment and reagents.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Schmidt, H., Mietlicki-Baase, E., Ige, K. et al. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacol 41, 1917–1928 (2016). https://doi.org/10.1038/npp.2015.362
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.362
This article is cited by
-
Neuroinflammatory Response in Reward-Associated Psychostimulants and Opioids: A Review
Cellular and Molecular Neurobiology (2023)
-
Liraglutide attenuates nicotine self-administration as well as nicotine seeking and hyperphagia during withdrawal in male and female rats
Psychopharmacology (2023)
-
Single nucleus transcriptomic analysis of rat nucleus accumbens reveals cell type-specific patterns of gene expression associated with volitional morphine intake
Translational Psychiatry (2022)
-
Novel Pharmacological Agents for the Treatment of Cocaine Use Disorder
Current Behavioral Neuroscience Reports (2022)
-
GLP-1 receptor signaling in the laterodorsal tegmental nucleus attenuates cocaine seeking by activating GABAergic circuits that project to the VTA
Molecular Psychiatry (2021)