Abstract
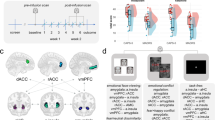
The neuropeptide oxytocin (OT) has been suggested as a promising pharmacological agent for medication-enhanced psychotherapy in posttraumatic stress disorder (PTSD) because of its anxiolytic and prosocial properties. We therefore investigated the behavioral and neurobiological effects of a single intranasal OT administration (40 IU) in PTSD patients. We conducted a randomized, placebo-controlled, cross-over resting-state fMRI study in male and female police officers with (n=37, 21 males) and without PTSD (n=40, 20 males). We investigated OT administration effects on subjective anxiety and functional connectivity of basolateral (BLA) and centromedial (CeM) amygdala subregions with prefrontal and salience processing areas. In PTSD patients, OT administration resulted in decreased subjective anxiety and nervousness. Under placebo, male PTSD patients showed diminished right CeM to left ventromedial prefrontal cortex (vmPFC) connectivity compared with male trauma-exposed controls, which was reinstated after OT administration. Additionally, female PTSD patients showed enhanced right BLA to bilateral dorsal anterior cingulate cortex (dACC) connectivity compared with female trauma-exposed controls, which was dampened after OT administration. Although caution is warranted, our findings tentatively suggest that OT has the potential to diminish anxiety and fear expression of the amygdala in PTSD, either via increased control of the vmPFC over the CeM (males) or via decreased salience processing of the dACC and BLA (females). Our findings add to accumulating evidence that OT administration could potentially enhance treatment response in PTSD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Acheson DT, Feifel D, Kamenski M, Mckinney R, Risbrough VB (2015). Intranasal oxytocin administration prior to exposure therapy for arachnophobia impedes treatment response. Depress Anxiety 32: 400–407.
American Psychiatrics Association (2013) DSM-V, Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing Inc.: Arlington, VA, USA.
Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ et al (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210: 343–352.
Bartz JA, Zaki J, Bolger N, Ochsner KN (2011). Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci 15: 301–309.
Bertsch K, Gamer M, Schmidt B, Schmidinger I, Walther S, Kästel T et al (2013). Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. Am J Psychiatry 170: 1169–1177.
Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS et al (1995). The development of a Clinician-Administered PTSD Scale. J Trauma Stress 8: 75–90.
Bradley R, Greene J, Russ E, Dutra L, Westen D (2005). A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry 162: 214–227.
Bremner JD, Bolus R, Mayer EA (2007). Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis 195: 211–218.
Brown VM, Labar KS, Haswell CC, Gold AL, Beall SK, Van Voorhees E et al (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39: 361–369.
Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A et al (2008a). Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med 38: 555–561.
Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A et al (2008b). Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp 29: 517–523.
Caldwell JD, Walker CH, Pedersen CA, Barakat AS, Mason GA (1994). Estrogen increases affinity of oxytocin receptors in the medial preoptic area-anterior hypothalamus. Peptides 15: 1079–1084.
Carlier I, Gersons B (1992). Development of a scale for traumatic incidents in police work. Psychiatr Fenn 23: 59–70.
Chao-Gan Y, Yu-Feng Z (2010). DPARSF: a MATLAB toolbox for ‘pipeline’ data analysis of resting-state fMRI. Front Syst Neurosci 4: 13.
de Kleine RA, Rothbaum BO, van Minnen A (2013). Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. Eur J Psychotraumatol 4.
de Oliveira DCG, Zuardi AW, Graeff FG, Queiroz RHC, Crippa JAS (2012). Anxiolytic-like effect of oxytocin in the simulated public speaking test. J Psychopharmacol 26: 497–504.
de Vries G, Olff M (2009). The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. J Trauma Stress 22: 259–267.
Dodhia S, Hosanagar A, Fitzgerald DA, Labuschagne I, Wood AG, Nathan PJ et al (2014). Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 39: 2061–2069.
Eckstein M, Becker B, Scheele D, Scholz C, Preckel K, Schlaepfer TE et al (2014). Oxytocin facilitates the extinction of conditioned fear in humans. Biol Psychiatry 78: 194–202.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K et al (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335.
Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB et al (2012). Neural correlates of attention bias to threat in post-traumatic stress disorder. Biol Psychol 90: 134–142.
Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B et al (2010). Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry 68: 678–680.
Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A et al (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci 18: 127–129.
First MB, Spitzer RL, Gibbon M, Williams JB. (2012) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)s, Clinician Version, Administration Booklet American Psychiatric Publishing Inc.: Arlington, VA.
Foa EB, Keane TM, Friedman MJ, Cohen JA (2009) Effective Treatments for PTSD: Practice Guidelines from the International Society of Traumatic Stress Studies. Guilford Press: New York, NY, USA.
Hayes JP, Hayes SM, Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2: 9.
Jovanovic T, Ressler KJ (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167: 648–662.
Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S et al (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25: 11489–11493.
Koch SB, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2015). Intranasal oxytocin administration dampens amygdala reactivity towards emotional faces in male and female PTSD patients. Neuropsychopharmacology (doi:10.1038/npp.2015.299; e-pub ahead of print).
Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2014). Intranasal oxytocin as strategy for medication-enhanced psychotherapy of PTSD: salience processing and fear inhibition processes. Psychoneuroendocrinology 40: 242–256.
Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M et al (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35: 2403–2413.
LeDoux J (1998). Fear and the brain: where have we been, and where are we going? Biol Psychiatry 44: 1229–1238.
Li Y, Qin W, Jiang T, Zhang Y, Yu C (2012). Sex-dependent correlations between the personality dimension of harm avoidance and the resting-state functional connectivity of amygdala subregions. PLoS One 7: e35925.
Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M et al (2012). Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology 37: 1431–1438.
Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK et al (2006). Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behav Neurosci 120: 1196–1203.
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454.
Nawijn L, van Zuiden M, Koch SBJ, Frijling JL, Veltman DJ, Olff M (2016a). Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder patients and traumatized controls. Psychoneuroendocrinology.
Nawijn L, van Zuiden M, Koch SBJ, Frijling JL, Veltman DJ, Olff M (2016b). Intranasal oxytocin increases insula responses to social reward in post-traumatic stress disorder.
Olff M, Frijling JL, Kubzansky LD, Bradley B, Ellenbogen MA, Cardoso C et al (2013). The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology 38: 1883–1894.
Olff M, Langeland W, Witteveen A, Denys D (2010). A psychobiological rationale for oxytocin in the treatment of posttraumatic stress disorder. CNS Spectr 15: 522–530.
Paloyelis Y, Doyle OM, Zelaya FO, Maltezos S, Williams SC, Fotopoulou A et al (2014). A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biol Psychiatry (doi:10.1016/j.biopsych.2014.10.005; e-pub ahead of print).
Patel R, Spreng RN, Shin LM, Girard TA (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–2142.
Rothbaum BO, Davis M (2003). Applying learning principles to the treatment of post-trauma reactions. Ann NY Acad Sci 1008: 112–121.
Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K et al (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59 Suppl 2: 22–33; quiz 34–57.
Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG (2013). Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol 16: 255–260.
Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC et al (2012a). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37: 241–249.
Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS et al (2012b). Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 74: 904–911.
Stefanacci L, Amaral DG (2002). Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol 451: 301–323.
Striepens N, Kendrick KM, Hanking V, Landgraf R, Wüllner U, Maier W et al (2013). Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep 3: 3440.
van Rooij SJH, Kennis M, Vink M, Geuze E (2015). Predicting treatment outcome in PTSD: a longitudinal functional MRI study on trauma-unrelated emotional processing. Neuropsychopharmacology (e-pub ahead of print).
Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A et al (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76: 183–201.
Zoicas I, Slattery DA, Neumann ID (2014). Brain oxytocin in social fear conditioning and its extinction: involvement of the lateral septum. Neuropsychopharmacology 39: 3027–3035.
Acknowledgements
We thank all participants for their participation in this study. We also thank Renée Hutter, Gré Westerveld, Marthe Hoofwijk, and all other personnel of the PDC police outpatient clinic for their valuable help with recruitment of the PTSD patients. This study is registered in the Netherlands Trial Registry (name: ‘The effect of oxytocin on brain processes in PTSD’, number: NTR3516, http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=3516).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Koch, S., van Zuiden, M., Nawijn, L. et al. Intranasal Oxytocin Normalizes Amygdala Functional Connectivity in Posttraumatic Stress Disorder. Neuropsychopharmacol 41, 2041–2051 (2016). https://doi.org/10.1038/npp.2016.1
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.1
This article is cited by
-
Central stress pathways in the development of cardiovascular disease
Clinical Autonomic Research (2024)
-
Oxytocin vs. placebo effects on intrusive memory consolidation using a trauma film paradigm: a randomized, controlled experimental study in healthy women
Translational Psychiatry (2023)
-
Oxytocin and orexin systems bidirectionally regulate the ability of opioid cues to bias reward seeking
Translational Psychiatry (2022)
-
In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing
Translational Psychiatry (2021)
-
Anterior prefrontal brain activity during emotion control predicts resilience to post-traumatic stress symptoms
Nature Human Behaviour (2021)