Abstract
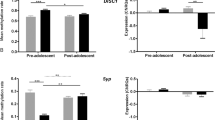
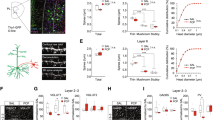
Schizophrenia (SZ) is a neurodevelopmental disorder in which the emergence of cognitive symptoms occurs during early adolescence. Glycogen synthase kinase-3β (GSK3β) plays a critical role in synaptic plasticity during development and is highly implicated in the etiology of SZ. However, how GSK3β activity affects synaptic plasticity and working memory function in the prefrontal cortex (PFC) during development remains unknown. Here we show a GSK3β hyperactivity during the early postnatal period in a neurodevelopmental rat SZ model that receives gestational exposure (E17) to the neurotoxin methylazoxymethanol (MAM). Accompanied with this change, adult MAM rats exhibited a significant decrease in spine density as well as impaired working memory, which was rescued by treatment with a GSK3β inhibitor during the juvenile period. Furthermore, the age-dependent hyperactive GSK3β caused a significant deficit in long-term potentiation (LTP) and facilitated long-term depression (LTD) in PFC pyramidal neurons. Notably, these changes in synaptic plasticity occurred only during the late juvenile period and were efficiently reversed by application of GSK3β inhibitors. Because the balance of LTP and LTD plays a critical role in activity-dependent synaptic stabilization and elimination during cortical development, the transient hyperactive GSK3β likely accounts for the cortical spine loss and PFC-dependent cognitive deficits in adulthood. These results highlight the importance of the postnatal trajectory of GSK3β for spine development and PFC function, and may shed light on the prophylactic treatment of cognitive symptoms in the SZ.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM (2008). Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci USA 105: 3123–3127.
Beurel E, Mines MA, Song L, Jope RS (2012). Glycogen synthase kinase-3 levels and phosphorylation undergo large fluctuations in mouse brain during development. Bipolar Disord 14: 822–830.
Bourgeois JP, Goldman-Rakic PS, Rakic P (1994). Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex 4: 78–96.
Bourne J, Harris KM (2007). Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17: 381–386.
Broadbelt K, Byne W, Jones LB (2002). Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res 58: 75–81.
Carpenter WT, Koenig JI (2008). The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology 33: 2061–2079.
Caruana DA, Warburton EC, Bashir ZI (2011). Induction of activity-dependent LTD requires muscarinic receptor activation in medial prefrontal cortex. J Neurosci 31: 18464–18478.
Collingridge GL, Peineau S, Howland JG, Wang YT (2010). Long-term depression in the CNS. Nat Rev Neurosci 11: 459–473.
Cuesto G, Enriquez-Barreto L, Carames C, Cantarero M, Gasull X, Sandi C et al (2011). Phosphoinositide-3-kinase activation controls synaptogenesis and spinogenesis in hippocampal neurons. J Neurosci 31: 2721–2733.
De Roo M, Klauser P, Muller D (2008). LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol 6: e219.
Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA (2004). Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet 36: 131–137.
Flagstad P, Glenthoj BY, Didriksen M (2005). Cognitive deficits caused by late gestational disruption of neurogenesis in rats: a preclinical model of schizophrenia. Neuropsychopharmacology 30: 250–260.
Franklin AV, King MK, Palomo V, Martinez A, McMahon LL, Jope RS (2014). Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biol Psychiatry 75: 198–206.
Freyberg Z, Ferrando SJ, Javitch JA (2010). Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167: 388–396.
Glantz LA, Lewis DA (2000). Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57: 65–73.
Glausier JR, Lewis DA (2013). Dendritic spine pathology in schizophrenia. Neuroscience 251: 90–107.
Hooper C, Markevich V, Plattner F, Killick R, Schofield E, Engel T et al (2007). Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. Eur J Neurosci 25: 81–86.
Insel TR (2010). Rethinking schizophrenia. Nature 468: 187–193.
King MK, Pardo M, Cheng Y, Downey K, Jope RS, Beurel E (2014). Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol Ther 141: 1–12.
Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R (2012). Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 109 (Suppl 2): 17186–17193.
Kozlovsky N, Belmaker RH, Agam G (2002). GSK-3 and the neurodevelopmental hypothesis of schizophrenia. Eur Neuropsychopharmacol 12: 13–25.
Leroy K, Brion JP (1999). Developmental expression and localization of glycogen synthase kinase-3beta in rat brain. J Chem Neuroanat 16: 279–293.
Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA (2007). Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol 10: 7–19.
Li YC, Xi D, Roman J, Huang YQ, Gao WJ (2009). Activation of glycogen synthase kinase-3 beta is required for hyperdopamine and D2 receptor-mediated inhibition of synaptic NMDA receptor function in the rat prefrontal cortex. J Neurosci 29: 15551–15563.
Lodge DJ, Grace AA (2009). Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res 204: 306–312.
Lovestone S, Killick R, Di Forti M, Murray R (2007). Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci 30: 142–149.
Mahmmoud RR, Sase S, Aher YD, Sase A, Groger M, Mokhtar M et al (2015). Spatial and working memory is linked to spine density and mushroom spines. PLoS One 10: e0139739.
Matsuo N, Reijmers L, Mayford M (2008). Spine-type-specific recruitment of newly synthesized AMPA receptors with learning. Science 319: 1104–1107.
Nadri C, Lipska BK, Kozlovsky N, Weinberger DR, Belmaker RH, Agam G (2003). Glycogen synthase kinase (GSK)-3beta levels and activity in a neurodevelopmental rat model of schizophrenia. Brain Res Dev Brain Res 141: 33–37.
Nagerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T (2004). Bidirectional activity-dependent morphological plasticity in hippocampal neurons. Neuron 44: 759–767.
Nelson CD, Kim MJ, Hsin H, Chen Y, Sheng M (2013). Phosphorylation of threonine-19 of PSD-95 by GSK-3beta is required for PSD-95 mobilization and long-term depression. J Neurosci 33: 12122–12135.
Ochs SM, Dorostkar MM, Aramuni G, Schon C, Filser S, Poschl J et al (2015). Loss of neuronal GSK3beta reduces dendritic spine stability and attenuates excitatory synaptic transmission via beta-catenin. Mol Psychiatry 20: 482–489.
Peineau S, Nicolas CS, Bortolotto ZA, Bhat RV, Ryves WJ, Harwood AJ et al (2009). A systematic investigation of the protein kinases involved in NMDA receptor-dependent LTD: evidence for a role of GSK-3 but not other serine/threonine kinases. Mol Brain 2: 22.
Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J et al (2007). LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron 53: 703–717.
Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM (2011). Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14: 285–293.
Peters A, Kaiserman-Abramof IR (1970). The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat 127: 321–355.
Sala C, Segal M (2014). Dendritic spines: the locus of structural and functional plasticity. Physiol Rev 94: 141–188.
Sanderson TM, Cotel MC, O'Neill MJ, Tricklebank MD, Collingridge GL, Sher E (2012). Alterations in hippocampal excitability, synaptic transmission and synaptic plasticity in a neurodevelopmental model of schizophrenia. Neuropharmacology 62: 1349–1358.
Selemon LD (2013). A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 3: e238.
Snyder MA, Adelman AE, Gao WJ (2013). Gestational methylazoxymethanol exposure leads to NMDAR dysfunction in hippocampus during early development and lasting deficits in learning. Neuropsychopharmacology 38: 328–340.
Spear LP (2000). The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463.
Stip E, Dufresne J, Lussier I, Yatham L (2000). A double-blind, placebo-controlled study of the effects of lithium on cognition in healthy subjects: mild and selective effects on learning. J Affect Disord 60: 147–157.
Tamura M, Mukai J, Gordon JA, Gogos JA (2016). Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron 89: 1100–1109.
Toyoda H, Zhao MG, Zhuo M (2005). Roles of NMDA receptor NR2A and NR2B subtypes for long-term depression in the anterior cingulate cortex. Eur J Neurosci 22: 485–494.
Wang HX, Gao WJ (2009). Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology 34: 2028–2040.
Wiegert JS, Oertner TG (2013). Long-term depression triggers the selective elimination of weakly integrated synapses. Proc Natl Acad Sci USA 110: E4510–E4519.
Wingo AP, Wingo TS, Harvey PD, Baldessarini RJ (2009). Effects of lithium on cognitive performance: a meta-analysis. J Clin Psychiatry 70: 1588–1597.
Xi D, Li YC, Snyder MA, Gao RY, Adelman AE, Zhang W et al (2011). Group II metabotropic glutamate receptor agonist ameliorates MK801-induced dysfunction of NMDA receptors via the Akt/GSK-3beta pathway in adult rat prefrontal cortex. Neuropsychopharmacology 36: 1260–1274.
Zhou Q, Homma KJ, Poo MM (2004). Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron 44: 749–757.
Zhu LQ, Liu D, Hu J, Cheng J, Wang SH, Wang Q et al (2010). GSK-3 beta inhibits presynaptic vesicle exocytosis by phosphorylating P/Q-type calcium channel and interrupting SNARE complex formation. J Neurosci 30: 3624–3633.
Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC et al (2007). Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci 27: 12211–12220.
Acknowledgements
We thank Miss Yelena Gulchina and Miss Sarah Monaco for their thoughtful comments and editorial work. BX designed and carried out all experiments and data analysis of western blots, electrophysiological recordings, morphological analysis, and behavioral tests. He wrote the paper. YCL helped electrophysiological recording and read the manuscript. WJG conceived the study, supervised the project, and finalized the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Xing, B., Li, YC. & Gao, WJ. GSK3β Hyperactivity during an Early Critical Period Impairs Prefrontal Synaptic Plasticity and Induces Lasting Deficits in Spine Morphology and Working Memory. Neuropsychopharmacol 41, 3003–3015 (2016). https://doi.org/10.1038/npp.2016.110
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.110
This article is cited by
-
Akt signaling pathway: a potential therapy for Alzheimer’s disease through glycogen synthase kinase 3 beta inhibition
The Egyptian Journal of Neurology, Psychiatry and Neurosurgery (2023)