Abstract
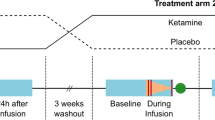
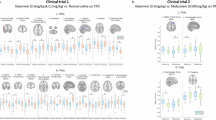
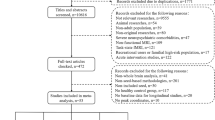
Capitalizing on recent advances in resting-state functional connectivity magnetic resonance imaging (rs-fcMRI) and the distinctive paradigm of rapid mood normalization following ketamine treatment, the current study investigated intrinsic brain networks in major depressive disorder (MDD) during a depressive episode and following treatment with ketamine. Medication-free patients with MDD and healthy control subjects (HC) completed baseline rs-fcMRI. MDD patients received a single infusion of ketamine and underwent repeated rs-fcMRI at 24 h posttreatment. Global brain connectivity with global signal regression (GBCr) values were computed as the average of correlations of each voxel with all other gray matter voxels in the brain. MDD group showed reduced GBCr in the prefrontal cortex (PFC) but increased GBCr in the posterior cingulate, precuneus, lingual gyrus, and cerebellum. Ketamine significantly increased GBCr in the PFC and reduced GBCr in the cerebellum. At baseline, 2174 voxels of altered GBCr were identified, but only 310 voxels significantly differed relative to controls following treatment (corrected α<0.05). Responders to ketamine showed increased GBCr in the lateral PFC, caudate, and insula. Follow-up seed-based analyses illustrated a pattern of dysconnectivity between the PFC/subcortex and the rest of the brain in MDD, which appeared to normalize postketamine. The extent of the functional dysconnectivity identified in MDD and the swift and robust normalization following treatment suggest that GBCr may serve as a treatment response biomarker for the development of rapid acting antidepressants. The data also identified unique prefrontal and striatal circuitry as a putative marker of successful treatment and a target for antidepressants’ development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL et al (2014). Glutamate metabolism in major depressive disorder. Am J Psychiatry 171: 1320–1327.
Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015). Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66: 509–523.
Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR et al (2013). Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry 73: 565–573.
Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH (2012). The role of default network deactivation in cognition and disease. Trends Cogn Sci 16: 584–592.
Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y et al (2015a). N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77: 569–580.
Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S et al (2014). Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biol Psychiatry 75: 595–605.
Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F et al (2015b). Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci 35: 267–286.
Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA et al (2009). The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14: 764–773739.
Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR et al (2013). The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage 83: 550–558.
Bobo WV, Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA (2016). Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety 33: 698–710.
Bullmore E, Sporns O (2009). Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10: 186–198.
Cole MW, Anticevic A, Repovs G, Barch D (2011). Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry 70: 43–50.
Cole MW, Pathak S, Schneider W (2010). Identifying the brain's most globally connected regions. Neuroimage 49: 3132–3148.
Cole MW, Yarkoni T, Repovs G, Anticevic A, Braver TS (2012). Global connectivity of prefrontal cortex predicts cognitive control and intelligence. J Neurosci 32: 8988–8999.
Drevets WC, Price JL, Furey ML (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213: 93–118.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D'Souza DC et al (2013a). Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry 18: 1199–1204.
Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D'Souza DC et al (2013b). The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology 38: 2613–2622.
Duman RS (2014). Pathophysiology of depression and innovative treatments: remodeling glutamatergic synaptic connections. Dialogues Clin Neurosci 16: 11–27.
Duman RS, Aghajanian GK (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science 338: 68–72.
Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS et al (2013). Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol 16: 301–311.
Fan N, Xu K, Ning Y, Rosenheck R, Wang D, Ke X et al (2016). Profiling the psychotic, depressive and anxiety symptoms in chronic ketamine users. Psychiatry Res 237: 311–315.
Gorman JM, Docherty JP (2010). A hypothesized role for dendritic remodeling in the etiology of mood and anxiety disorders. J Neuropsychiatry Clin Neurosci 22: 256–264.
Hyder F, Rothman DL, Bennett MR (2013). Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. Proc Natl Acad Sci USA 110: 3549–3554.
Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015). Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72: 603–611.
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P et al (2012). Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18: 1413–1417.
Koechlin E, Corrado G, Pietrini P, Grafman J (2000). Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci USA 97: 7651–7656.
Krystal JH, Anticevic A (2015). Toward illness phase-specific pharmacotherapy for schizophrenia. Biol Psychiatry 78: 738–740.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69: 754–761.
Liang X, Connelly A, Calamante F (2014). Graph analysis of resting-state ASL perfusion MRI data: nonlinear correlations among CBF and network metrics. Neuroimage 87: 265–275.
Liang X, Zou Q, He Y, Yang Y (2013). Coupling of functional connectivity and regional cerebral blood flow reveals a physiological basis for network hubs of the human brain. Proc Natl Acad Sci USA 110: 1929–1934.
MacQueen G, Frodl T (2011). The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 16: 252–264.
Mayberg HS (2003). Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65: 193–207.
Menon V (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15: 483–506.
Morgan CJ, Curran HV, Independent Scientific Committee on Drugs (2012). Ketamine use: a review. Addiction 107: 27–38.
Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ et al (2015). Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry 5: e509.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM et al (2013). Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 170: 1134–1142.
Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E et al (2005). Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am J Psychiatry 162: 394–396.
Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H et al (2012). Ketamine decreases resting state functional network connectivity in healthy subjects: implications for antidepressant drug action. PLoS One 7: e44799.
Sen S, Duman R, Sanacora G (2008). Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 64: 527–532.
Sheline YI, Price JL, Yan Z, Mintun MA (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 107: 11020–11025.
Stone JM, Dietrich C, Edden R, Mehta MA, De Simoni S, Reed LJ et al (2012). Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry 17: 664–665.
Tomasi D, Wang GJ, Volkow ND (2013). Energetic cost of brain functional connectivity. Proc Natl Acad Sci USA 110: 13642–13647.
Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163: 28–40.
Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103: 297–321.
Wang JX, Voss JL (2014). Brain networks for exploration decisions utilizing distinct modeled information types during contextual learning. Neuron 82: 1171–1182.
Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W et al (2015). The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum Brain Mapp 36: 768–778.
Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z (2009). Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA 106: 14075–14079.
Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS et al (2011). Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry 16: 156–170.
Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73: 962–977.
Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA et al (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63: 856–864.
Acknowledgements
We thank all the study participants for their invaluable contribution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Abdallah, C., Averill, L., Collins, K. et al. Ketamine Treatment and Global Brain Connectivity in Major Depression. Neuropsychopharmacol 42, 1210–1219 (2017). https://doi.org/10.1038/npp.2016.186
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.186
This article is cited by
-
The relationship between vitamin D levels and depression: a genetically informed study
Nutrition Journal (2025)
-
Challenges and rewards of in vivo synaptic density imaging, and its application to the study of depression
Neuropsychopharmacology (2025)
-
Psychedelics and ketamine/esketamine in depressive disorders: biological mechanisms and associated neuroimaging and clinical changes
Translational Psychiatry (2025)
-
Rapid antidepressant potential of nitrous oxide: current state and major questions
Molecular Psychiatry (2025)
-
Convergent functional effects of antidepressants in major depressive disorder: a neuroimaging meta-analysis
Molecular Psychiatry (2025)