Abstract
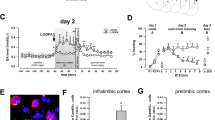
Emerging evidence supports a role for dopamine in major depressive disorder (MDD). We recently reported fewer spontaneously active ventral tegmental area (VTA) dopamine neurons (ie, reduced dopamine neuron population activity) in the chronic mild stress (CMS) rodent model of MDD. In this study, we examined the role of two brain regions that have been implicated in MDD in humans, the infralimbic prefrontal cortex (ILPFC)—that is, rodent homolog of Brodmann area 25 (BA25), and the lateral habenula (LHb) in the CMS-induced attenuation of dopamine neuron activity. The impact of activating the ILPFC or LHb was evaluated using single-unit extracellular recordings of identified VTA dopamine neurons. The involvement of each region in dopamine neuron attenuation following 5–7 weeks of CMS was then evaluated by selective inactivation. Activation of either ILPFC or LHb in normal rats potently suppressed dopamine neuron population activity, but in unique patterns. ILPFC activation selectively inhibited dopamine neurons in medial VTA, which were most impacted by CMS. Conversely, LHb activation selectively inhibited dopamine neurons in lateral VTA, which were unaffected by CMS. Moreover, only ILPFC inactivation restored dopamine neuron population activity to normal levels following CMS; LHb inactivation had no restorative effect. These data suggest that, in the CMS model of MDD, the ILPFC is the primary driver of diminished dopamine neuron responses. These findings support a neural substrate for ILPFC/BA25 linking affective and motivational circuitry dysfunction in MDD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Belujon P, Grace AA (2014). Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76: 927–936.
Belujon P, Jakobowski NL, Dollish HK, Grace AA (2016). Withdrawal from acute amphetamine induces an amygdala-driven attenuation of dopamine neuron activity: reversal by ketamine. Neuropsychopharmacology 41: 619–627.
Carlsson A (1976). The contribution of drug research to investigating the nature of endogenous depression. Pharmakopsychiatr Neuro-Psychopharmakol 9: 2–10.
Chang CH, Grace AA (2013). Amygdala beta-noradrenergic receptors modulate delayed downregulation of dopamine activity following restraint. J Neurosci 33: 1441–1450.
Chang CH, Grace AA (2014). Amygdala–ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biol Psychiatry 76: 223–230.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493: 532–536.
Clark L, Chamberlain SR, Sahakian BJ (2009). Neurocognitive mechanisms in depression: implications for treatment. Annu Rev Neurosci 32: 57–74.
Drevets WC (1999). Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci 877: 614–637.
Eshel N, Tian J, Bukwich M, Uchida N (2016). Dopamine neurons share common response function for reward prediction error. Nat Neurosci 19: 479–486.
Ferenczi EA, Zalocusky KA, Liston C, Grosenick L, Warden MR, Amatya D et al (2016). Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science (New York, NY) 351: aac9698.
Floresco SB, Todd CL, Grace AA (2001). Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci 21: 4915–4922.
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973.
Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J et al (2014). Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science (New York, NY) 344: 313–319.
Grace AA (2016). Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci 17: 524–532.
Grace AA, Bunney BS (1983). Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience 10: 301–315.
Grace AA, Bunney BS (1984a). The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci 4: 2877–2890.
Grace AA, Bunney BS (1984b). The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci 4: 2866–2876.
Grace AA, Floresco SB, Goto Y, Lodge DJ (2007). Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30: 220–227.
Heilbronner SR, Rodriguez-Romaguera J, Quirk GJ, Groenewegen HJ, Haber SN (2016). Circuit-based corticostriatal homologies between rat and primate. Biol Psychiatry 80: 509–521.
Henn FA, Vollmayr B (2005). Stress models of depression: forming genetically vulnerable strains. Neurosci Biobehav Rev 29: 799–804.
Hikosaka O (2010). The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci 11: 503–513.
Hikosaka O, Sesack SR, Lecourtier L, Shepard PD (2008). Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci 28: 11825–11829.
Ikemoto S (2007). Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27–78.
Ji H, Shepard PD (2007). Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci 27: 6923–6930.
Koob GF, Stinus L, Le Moal M, Bloom FE (1989). Opponent process theory of motivation: neurobiological evidence from studies of opiate dependence. Neurosci Biobehav Rev 13: 135–140.
Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM et al (2012). Input-specific control of reward and aversion in the ventral tegmental area. Nature 491: 212–217.
Lawson RP, Drevets WC, Roiser JP (2013). Defining the habenula in human neuroimaging studies. NeuroImage 64: 722–727.
Lawson RP, Nord CL, Seymour B, Thomas DL, Dayan P, Pilling S et al (2016). Disrupted habenula function in major depression. Mol Psychiatry (e-pub ahead of print).
Li B, Piriz J, Mirrione M, Chung C, Proulx CD, Schulz D et al (2011). Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature 470: 535–539.
Lodge DJ, Grace AA (2006). The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology 31: 1356–1361.
Mayberg HS (2003). Modulating dysfunctional limbic–cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65: 193–207.
Mayberg HS (2009). Targeted electrode-based modulation of neural circuits for depression. J Clin Invest 119: 717–725.
Moore H, Rose HJ, Grace AA (2001). Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology 24: 410–419.
Nestler EJ, Carlezon WA Jr (2006). The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59: 1151–1159.
Patton MH, Bizup BT, Grace AA (2013). The infralimbic cortex bidirectionally modulates mesolimbic dopamine neuron activity via distinct neural pathways. J Neurosci 33: 16865–16873.
Ressler KJ, Nemeroff CB (2000). Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress anxiety 12 (Suppl 1): 2–19.
Salamone JD, Correa M (2012). The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 470–485.
Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW et al (2010). Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry 67: e9–e11.
Slattery DA, Cryan JF (2012). Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protocols 7: 1009–1014.
Stamatakis AM, Stuber GD (2012). Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci 15: 1105–1107.
Stopper CM, Floresco SB (2014). What's better for me? Fundamental role for lateral habenula in promoting subjective decision biases. Nat Neurosci 17: 33–35.
Treadway MT, Zald DH (2011). Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev 35: 537–555.
Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J et al (2013). Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 493: 537–541.
Ungless MA, Grace AA (2012). Are you or aren't you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci 35: 422–430.
Valenti O, Gill KM, Grace AA (2012). Different stressors produce excitation or inhibition of mesolimbic dopamine neuron activity: response alteration by stress pre-exposure. Eur J Neurosci 35: 1312–1321.
Valenti O, Lodge DJ, Grace AA (2011). Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci 31: 4280–4289.
Weiss JM (1997). Does decreased sucrose intake indicate loss of preference in CMS model? Psychopharmacology 134: 368–370.
Willner P, Lappas S, Cheeta S, Muscat R (1994). Reversal of stress-induced anhedonia by the dopamine receptor agonist, pramipexole. Psychopharmacology 115: 454–462.
Willner P, Muscat R, Papp M (1992). Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev 16: 525–534.
Zimmerman EC, Grace AA (2016). The nucleus reuniens of the midline thalamus gates prefrontal-hippocampal modulation of ventral tegmental area dopamine neuron activity. J Neurosci 36: 8977–8984.
Acknowledgements
We thank Kathryn Gill, Pauline Belujon, Millie Rincon-Cortes, Felipe Gomes, and Tomek Banasikowski for helpful discussion and guidance, and Niki MacMurdo and Christy Smolak for histology technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Moreines, J., Owrutsky, Z. & Grace, A. Involvement of Infralimbic Prefrontal Cortex but not Lateral Habenula in Dopamine Attenuation After Chronic Mild Stress. Neuropsychopharmacol 42, 904–913 (2017). https://doi.org/10.1038/npp.2016.249
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.249
This article is cited by
-
Adult stress exposure blunts dopamine system hyperresponsivity in a neurodevelopmental rodent model of schizophrenia
Schizophrenia (2022)
-
Nucleus reuniens inactivation reverses stress-induced hypodopaminergic state and altered hippocampal-accumbens synaptic plasticity
Neuropsychopharmacology (2022)
-
Postpartum scarcity-adversity disrupts maternal behavior and induces a hypodopaminergic state in the rat dam and adult female offspring
Neuropsychopharmacology (2022)
-
The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders
Nature Reviews Neuroscience (2022)
-
Prefrontal cortex and depression
Neuropsychopharmacology (2022)