Abstract
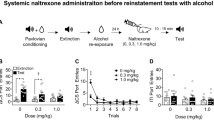
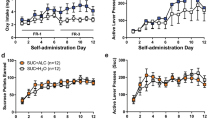
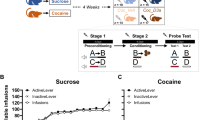
Varenicline, a pharmacotherapy for tobacco addiction, reduces alcohol consumption in humans and rodents. The therapeutic potential of varenicline would escalate if it also diminished conditioned responses elicited by alcohol-predictive cues, which can precipitate relapse in abstinent individuals. We investigated this application, along with the underlying neural substrates, using a robust preclinical assay in which relapse to alcohol-seeking was triggered by re-exposure to an alcohol-associated environmental context. Male, Long-Evans rats received Pavlovian conditioning sessions in which one auditory conditioned stimulus (CS+) was paired with 15% ethanol and a second conditioned stimulus (CS−) was not. Ethanol was delivered into a port for oral consumption and port entries triggered by each CS were recorded. Extinction was then conducted in a different context where the CS+ and CS− were presented without ethanol. To stimulate relapse, both cues were subsequently presented without ethanol in the prior conditioning context. Systemic varenicline (0, 0.5 or 2.5 mg/kg; intraperitoneal) blocked context-induced relapse to alcohol-seeking without affecting the ability to make a port entry. It also reduced context-induced relapse to sucrose-seeking, but only at the 2.5 mg/kg dose. Neuropharmacological studies showed that context-induced relapse to alcohol-seeking was attenuated by bilateral microinfusion of varenicline (0.3 μl/side) into the nucleus accumbens (NAc; 0 or 3.5 μg), but not the ventral tegmental area (0, 2 or 4 μg). These data show for the first time that varenicline reduces relapse triggered by contexts that predict alcohol, and suggest that nicotinic acetylcholine receptors in the NAc are critical for this effect.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bossert JM, Liu SY, Lu L, Shaham Y (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci 24: 10726–10730.
Burattini C, Gill TM, Aicardi G, Janak PH (2006). The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience 139: 877–887.
Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M et al (2012). Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep 2: 33–41.
Caggiula AR, Donny EC, Chaudhri N, Perkins KA, Evans-Martin FF, Sved AF (2002). Importance of nonpharmacological factors in nicotine self-administration. Physiol Behav 77: 683–687.
Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Léna C et al (2003). Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci 23: 7820–7829.
Chaudhri N, Sahuque L, Janak P (2008a). Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry 64: 203–210.
Chaudhri N, Sahuque LL, Cone JJ, Janak PH (2008b). Reinstated ethanol-seeking in rats is modulated by environmental context and requires the nucleus accumbens core. Eur J Neurosci 28: 2288–2298.
Chaudhri N, Sahuque LL, Janak PH (2009). Ethanol seeking triggered by environmental context is attenuated by blocking dopamine D1 receptors in the nucleus accumbens core and shell in rats. Psychopharmacology (Berl) 207: 303–314.
Chaudhri N, Sahuque LL, Schairer WW, Janak P (2010). Separable roles of the nucleus accumbens core and shell in context- and cue-induced alcohol-seeking. Neuropsychopharmacology 35: 783–791.
Chaudhri N, Woods CA, Sahuque LL, Gill TM, Janak PH (2013). Unilateral inactivation of the basolateral amygdala attenuates context-induced renewal of Pavlovian-conditioned alcohol-seeking. Eur J Neurosci 38: 2751–2761.
Cortright JJ, Sampedro GR, Neugebauer NM, Vezina P (2012). Previous exposure to nicotine enhances the incentive motivational effects of amphetamine via nicotine-associated contextual stimuli. Neuropsychopharmacology 37: 2277–2284.
Crombag HS, Shaham Y (2002). Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behav Neurosci 116: 169–173.
de Bejczy A, Löf E, Walther L, Guterstam J, Hammarberg A, Asanovska G et al (2015). Varenicline for treatment of alcohol aependence: a randomized, placebo-controlled trial. Alcohol Clin Exp Res 39: 2189–2199.
Feduccia AA, Simms JA, Mill D, Yi HY, Bartlett SE (2014). Varenicline decreases ethanol intake and increases dopamine release via neuronal nicotinic acetylcholine receptors in the nucleus accumbens. Br J Pharmacol 171: 3420–3431.
Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O’Malley SS (2011). A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology (Berl) 215: 655–663.
Funk D, Lo S, Coen K, Lê AD (2016). Effects of varenicline on operant self-administration of alcohol and/or nicotine in a rat model of co-abuse. Behav Brain Res 296: 157–162.
Gowin JL, Vatsalya V, Westman JG, Schwandt ML, Bartlett SE, Heilig M et al (2016). The effect of varenicline on the neural processing of fearful faces and the subjective effects of alcohol in heavy drinkers. Alcohol Clin Exp Res 40: 979–987.
Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB et al (2010). Structural differences determine the relative selectivity of nicotinic compounds for native α4β2*-, α6β2*-, α3β4*- and α7-nicotine acetylcholine receptors. Neuropharmacology 58: 1054–1066.
Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh M, Collins AC (2002). Characterization of nicotinic agonist-induced [3H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther 301: 651–660.
Guillem K, Peoples LL (2010). Varenicline effects on cocaine self administration and reinstatement behavior. Behav Pharmacol 21: 96–103.
Hall BJ, Pearson LS, Buccafusco JJ (2010). Effect of administration of the nicotinic acetylcholine receptor antagonist BTMPS, during nicotine self-administration, on lever responding induced by context long after withdrawal. Neuropharmacology 58: 429–435.
Janak PH, Chaudhri N (2010). The potent effect of environmental context on relapse to alcohol-seeking after extinction. Open Addict J 3: 76–87.
Katner SN, Weiss F (1999). Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res 23: 1751–1760.
Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I et al (2011). Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 15: 1265–1274.
Litt MD, Cooney NL, Morse P (2000). Reactivity to alcohol-related stimuli in the laboratory and in the field: predictors of craving in treated alcoholics. Addiction 95: 889–900.
Litten RZ, Ryan ML, Fertig JB, Falk DE, Johnson B, Dunn KE et al (2013). A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med 7: 277–286.
Löf E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR et al (2007). Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 195: 333–343.
Marinelli PW, Funk D, Juzytsch W, Li Z, Lê AD (2007). Effects of opioid receptor blockade on the renewal of alcohol seeking induced by context: relationship to c-fos mRNA expression. Eur J Neurosci 26: 2815–2823.
McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM et al (2009). Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66: 185–190.
Mihalak KB, Carroll FI, Luetje CW (2006). Varenicline is a partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol 70: 801–805.
Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL (2012). Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology 223: 299–306.
O’Connor EC, Parker D, Rollema H, Mead AN (2009). The α4β2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology 208: 365–376.
Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego.
Rodd ZA, Melendez RL, Bell RL, Kuc KA, Zhang Y, Murphy JM et al (2004). Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci 24: 1050–1057.
Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ (2000). Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 149: 217–224.
Sajja RK, Rahman S (2013). Nicotinic receptor partial agonists modulate alcohol deprivation effect in C57BL/6J mice. Pharmacol Biochem Behav 110: 161–167.
Schacht J, Anton RF, RAndall PK, Li X, Henderson S, Myrick H (2014). Varenicline effects on drinking, craving and neural reward processing among non-treatment-seeking alcohol-dependent individuals. Psychopharmacology 231: 3799–3807.
Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R et al (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long–Evans and Wistar rats. Alcohol Clin Exp Res 32: 1816–1823.
Soyka M, Mutschler J (2015). Treatment-refractory substance use disorder: focus on alcohol, opioids, and cocaine. Prog Neuropsychopharmacol Biol Psychiatry 70: 148–161.
Sparks LM, Sciascia JM, Ayorech Z, Chaudhri N (2013). Vendor differences in alcohol consumption and the contribution of dopamine receptors to Pavlovian-conditioned alcohol-seeking in Long-Evans rats. Psychopharmacology 231: 753–764.
Steensland P, Simms JA, Holgate JK, Richards J, Bartlett SE (2007). Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci USA 104: 12518–12523.
Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ (2012). Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75: 58–64.
Wise RA (1973). Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 29: 203–210.
Wouda JA, Riga D, De Vries W, Stegeman M, van Mourik Y, Schetters D et al (2011). Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 216: 267–277.
Yoshida K, Engel J, Liljequist S (1982). The effect of chronic ethanol administration on high affinity3H-nicotinic binding in rat brain. Naunyn Schmiedeberg Arch Pharmacol 321: 74–76.
Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M et al (2007). Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience 144: 1209–1218.
Zironi I, Burattini C, Aicardi G, Janak PH (2006). Context is a trigger for relapse to alcohol. Behav Brain Res 167: 150–155.
Acknowledgements
Experiments were designed by NC and conducted by FL, AP and J-MM. FL, AP, and J-MM analyzed the data. AHJ contributed to implementing Experiment 1 and trained FL. FL and NC wrote the manuscript, and all authors reviewed the manuscript and approved the final submission. We thank Steve Cabilio and David Munro for technical support and Dang-Vinh Chi Vo for conducting an experiment that was included in the original manuscript submission but removed in the revision due to space constraints.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Lacroix, F., Pettorelli, A., Maddux, JM. et al. Varenicline Reduces Context-Induced Relapse to Alcohol-Seeking through Actions in the Nucleus Accumbens. Neuropsychopharmacol 42, 1037–1048 (2017). https://doi.org/10.1038/npp.2016.254
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.254
This article is cited by
-
Considering Drug-Associated Contexts in Substance Use Disorders and Treatment Development
Neurotherapeutics (2020)