Abstract
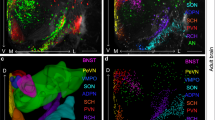
The neuropeptides oxytocin (OXT) and vasopressin (AVP) have been identified as modulators of emotional social behaviors and associated with neuropsychiatric disorders characterized by social dysfunction. Experimental and therapeutic use of OXT and AVP via the intranasal route is the subject of extensive clinical research. However, the large-scale functional substrates directly engaged by these peptides and their functional dynamics remain elusive. By using cerebral blood volume (CBV) weighted fMRI in the mouse, we show that intranasal administration of OXT rapidly elicits the transient activation of cortical regions and a sustained activation of hippocampal and forebrain areas characterized by high oxytocin receptor density. By contrast, intranasal administration of AVP produced a robust and sustained deactivation in cortico-parietal, thalamic and mesolimbic regions. Importantly, intravenous administration of OXT and AVP did not recapitulate the patterns of modulation produced by intranasal dosing, supporting a central origin of the observed functional changes. In keeping with this notion, hippocampal local field potential recordings revealed multi-band power increases upon intranasal OXT administration. We also show that the selective OXT-derivative TGOT reproduced the pattern of activation elicited by OXT and that the deletion of OXT receptors does not affect AVP-mediated deactivation. Collectively, our data document divergent modulation of brainwide neural systems by intranasal administration of OXT and AVP, an effect that involves key substrates of social and emotional behavior. The observed divergence calls for a deeper investigation of the systems-level mechanisms by which exogenous OXT and AVP modulate brain function and exert their putative therapeutic effects.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aguilera G, Pham Q, Rabadan C (1994). Regulation of pituitary vasopressin receptors during chronic stress: relationship to corticotroph responsiveness. J Neuroendocrinol 6: 299–304.
Anagnostou E, Soorya L, Brian J, Dupuis A, Mankad D, Smile S et al (2014). Intranasal oxytocin in the treatment of autism spectrum disorders: a review of literature and early safety and efficacy data in youth. Brain Res 1580: 188–198.
Bakermans-Kranenburg MJ, van IJzendoorn MH (2013). Sniffing around oxytocin: review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry 3: e258.
Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH et al (2014). Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry 4: e480.
Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM et al (2015). Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry 74: 180–188.
Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E (2008). Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron 58: 639–650.
Boccia MM, Baratti CM (2000). Involvement of central cholinergic mechanisms in the effects of oxytocin and an oxytocin receptor antagonist on retention performance in mice. Neurobiol Learn Mem 74: 217–228.
Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL (2002). Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci 5: 514–516.
Bosch OJ, Pförtsch J, Beiderbeck DI, Landgraf R, Neumann ID (2010). Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol 22: 420–429.
Busnelli M, Bulgheroni E, Manning M, Kleinau G, Chini B (2013). Selective and potent agonists and antagonists for investigating the role of mouse oxytocin receptors. J Pharmacol Exp Ther 346: 318–327.
Calcagnoli F, Kreutzmann JC, de Boer SF, Althaus M, Koolhaas JM (2015). Acute and repeated intranasal oxytocin administration exerts anti-aggressive and pro-affiliative effects in male rats. Psychoneuroendocrinology 51: 112–121.
Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF et al (2013). Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res 147: 393–397.
Dhuria SV, Hanson LR, Frey WH (2010). Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99: 1654–1673.
Diehl K-H, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D et al (2001). A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21: 15–23.
Dolen G, Darvishzadeh A, Huang KW, Malenka RC (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501: 179–184.
Donaldson ZR, Young LJ (2008). Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322: 900–904.
Donnelley M, Morgan KS, Fouras A, Skinner W, Uesugi K, Yagi N et al (2009). Real-time non-invasive detection of inhalable particulates delivered into live mouse airways. J Synchrotron Radiat 16: 553–561.
Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013). Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav 64: 693–701.
Ebstein RP, Israel S, Lerer E, Uzefovsky F, Shalev I, Gritsenko I et al (2009). Arginine vasopressin and oxytocin modulate human social behavior. Ann NY Acad Sci 1167: 87–102.
Errico F, D'Argenio V, Sforazzini F, Iasevoli F, Squillace M, Guerri G et al (2015). A role for D-aspartate oxidase in schizophrenia and in schizophrenia-related symptoms induced by phencyclidine in mice. Transl Psychiatry 5: e512.
Febo M, Numan M, Ferris CF (2005). Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci 25: 11637–11644.
Feifel D, Shilling PD, Macdonald K (2016). A review of oxytocin's effects on the positive, negative, and cognitive domains of schizophrenia. Biol Psychiatry 79: 222–233.
Ferrari L, Turrini G, Crestan V, Bertani S, Cristofori P, Bifone A et al (2012). A robust experimental protocol for pharmacological fMRI in rats and mice. J Neurosci Methods 204: 9–18.
Ferris CF (2008). Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. Prog Brain Res 170: 305–320.
Ferris CF, Yee J, Kenkel W, Dumais KM, Moore K, Veenema AH et al (2015). Distinct BOLD activation profiles following central and peripheral oxytocin administration in awake rats. Front Behav Neurosci 9: 245.
Freeman SM, Young LJ (2016). Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: Translational implications. J Neuroendocrinol 28: 1–12.
Gozzi A, Schwarz AJ (2015). Large-scale functional connectivity networks in the rodent brain. Neuroimage 127: 496–509.
Gozzi A, Schwarz AJ, Reese T, Crestan V, Bifone A (2008). Drug-anaesthetic interaction in phMRI: the case of the pyschotomimetic agent phencyclidine. Magn Reson Imaging 26: 999–1006.
Gozzi A, Ceolin L, Schwarz A, Reese T, Bertani S, Crestan V et al (2007). A multimodality investigation of cerebral hemodynamics and autoregulation in pharmacological MRI. Magn Reson Imaging 25: 826–833.
Gozzi A, Colavito V, Seke Etet PF, Montanari D, Fiorini S, Tambalo S et al (2012). Modulation of fronto-cortical activity by modafinil: a functional imaging and fos study in the rat. Neuropsychopharmacology 37: 822–837.
Gozzi A, Apar J, Giovanelli A, Bertollini C, Crestan V, Schwarz AJ et al (2010). A neural switch for active and passive fear. Neuron 67: 656–666.
Guastella AJ, Hickie IB (2016). Oxytocin treatment, circuitry and autism: a critical review of the literature placing oxytocin into the autism context. Biol Psychiatry 79: 234–242.
Hammock E, Levitt P (2013). Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6 J mouse. Front Behav Neurosci 7: 195.
Harkema JR, Carey SA, Wagner JG (2006). The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol 34: 252–269.
Henry RJ, Ruano N, Casto D, Wolf RH (1998). A pharmacokinetic study of midazolam in dogs: nasal drop vs. atomizer administration. Pediatr Dent 20: 321–326.
Hermes MLHJ, Ruijter JM, Klop A, Buijs RM, Renaud LP (2000). Vasopressin increases GABAergic inhibition of rat hypothalamic paraventricular nucleus neurons in vitro. J Neurophysiol 83: 705–711.
Hernando F, Schoots O, Lolait SJ, Burbach JP (2001). Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin 1. Endocrinology 142: 1659–1668.
Hoekman JD, Ho RJ (2011). Enhanced analgesic responses after preferential delivery of morphine and fentanyl to the olfactory epithelium in rats. Anesth Analg 113: 641.
Hofmann SG, Fang A, Brager DN (2015). Effect of intranasal oxytocin administration on psychiatric symptoms: a meta-analysis of placebo-controlled studies. Psychiatry Res 228: 708–714.
Huang H, Michetti C, Busnelli M, Manago F, Sannino S, Scheggia D et al (2014). Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology 39: 1102–1114.
Huber D, Veinante P, Stoop R (2005). Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 308: 245–248.
Insel TR (2010). The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron 65: 768–779.
Insel TR, O'Brien DJ, Leckman JF (1999). Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry 45: 145–157.
Jocham Radua MD, Shergill SS (2015). Neurophysiological effects of acute oxytocin administration: systematic review and meta-analysis of placebo-controlled imaging studies. J Psychiatry Neurosci 40: E1.
Kalisch R, Elbel GK, Gossl C, Czisch M, Auer DP (2001). Blood pressure changes induced by arterial blood withdrawal influence bold signal in anesthesized rats at 7 Tesla: implications for pharmacologic MRI. Neuroimage 14: 891–898.
Kirkpatrick MG, Lee R, Wardle MC, Jacob S, de Wit H (2014). Effects of MDMA and intranasal oxytocin on social and emotional processing. Neuropsychopharmacology 39: 1654–1663.
Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S et al (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci 25: 11489–11493.
Kosaki Y, Watanabe S (2016). Conditioned social preference, but not place preference, produced by intranasal oxytocin in female mice. Behav Neurosci 130: 182–195.
Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M et al (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology 35: 2403–2413.
Landgraf R, Ke+ƒler MS, Bunck M, Murgatroyd C, Spengler D, Zimbelmann M et al (2007). Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: focus on vasopressin and glyoxalase-I. Neurosci Biobehav Rev 31: 89–102.
Lee RJ, Coccaro EF, Cremers H, McCarron R, Lu SF, Brownstein MI et al (2013). A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: an fMRI study. Front Syst Neurosci 7: 100.
Leng G, Ludwig M (2016). Intranasal oxytocin: myths and delusions. Biol Psychiatry 79: 243–250.
Litvin Y, Murakami G, Pfaff DW (2011). Effects of chronic social defeat on behavioral and neural correlates of sociality: vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav 103: 393–403.
Logothetis NK (2008). What we can do and what we cannot do with fMRI. Nature 453: 869–878.
Ludwig M, Bull PM, Tobin VA, Sabatier N, Landgraf R, Dayanithi G et al (2005). Regulation of activity-dependent dendritic vasopressin release from rat supraoptic neurones. J Physiol 564: 515–522.
Ludwig M, Tobin VA, Callahan MF, Papadaki E, Becker A, Engelmann M et al (2013). Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats. J Neuroendocrinol 25: 655–667.
Lukas M, Neumann ID (2013). Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res 251: 85–94.
Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID (2011). The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology 36: 2159–2168.
Luo F, Wu G, Li Z, Li S-J (2003). Characterisation of effects of mean arterial blood pressure induced by cocaine and cocaine methiodide on BOLD signals in the rat brain. Magn Reson Med 49: 264–270.
Mahmud M, Pasqualotto E, Bertoldo A, Girardi S, Maschietto M, Vassanelli S (2011). An automated method for detection of layer activation order in information processing pathway of rat barrel cortex under mechanical whisker stimulation. J Neurosci Methods 196: 141–150.
Maier A, Wilke M, Aura C, Zhu C, Ye FQ, Leopold DA (2008). Divergence of fMRI and neural signals in V1 during perceptual suppression in the awake monkey. Nat Neurosci 11: 1193–1200.
Mandeville JB, Marota JJA, Kosofsky BE, Keltner JR, Weissleder R, Rosen B et al (1998). Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn Reson Med 39: 615–624.
Marlin BJ, Mitre M, D′amour JA, Chao MV, Froemke RC (2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520: 499–504.
McCarthy MT, McDonald BC, Brooks PJ, Goldman D (1996). An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiol Behav 60: 1209–1215.
Mens WBJ, Witter A, Van Wimersma Greidanus TB (1983). Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res 262: 143–149.
Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci 12: 524–538.
Modi ME, Connor-Stroud F, Landgraf R, Young LJ, Parr LA (2014). Aerosolized oxytocin increases cerebrospinal fluid oxytocin in rhesus macaques. Psychoneuroendocrinology 45: 49–57.
Neumann ID, Landgraf R (2012). Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35: 649–659.
Neumann ID, Slattery DA (2016). Oxytocin in general anxiety and social fear: a translational approach. Biol Psychiatry 79: 213–221.
Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R (2013). Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology 38: 1985–1993.
Orth M, Bravo E, Barter L, Carstens E, Antognini JF (2006). The differential effects of halothane and isoflurane on electroencephalographic responses to electrical microstimulation of the reticular formation. Anesth Analg 102: 1709–1714.
Ostrowski NL, Lolait SJ, Young WS 3rd (1994). Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology 135: 1511–1528.
Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW (2013). Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500: 458–462.
Paloyelis Y, Doyle OM, Zelaya FO, Maltezos S, Williams SC, Fotopoulou A et al (2014). A spatiotemporal profile of in vivo cerebral blood flow changes following intranasal oxytocin in humans. Biol Psychiatry 79: 693–705.
Penagarikano O, Lzaro MT, Lu XH, Gordon A, Dong H, Lam HA et al (2015). Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med 7: 271ra278–271ra278.
Raggenbass M (2001). Vasopressin- and oxytocin-induced activity in the central nervous system: electrophysiological studies using in-vitro systems. Prog Neurobiol 64: 307–326.
Ramos L, Hicks C, Caminer A, McGregor IS (2014). Inhaled vasopressin increases sociability and reduces body temperature and heart rate in rats. Psychoneuroendocrinology 46: 46–51.
Riem MME, Bakermans-Kranenburg MJ, Voorthuis A, van IJzendoorn MH (2014). Oxytocin effects on mind-reading are moderated by experiences of maternal love withdrawal: an fMRI study. Prog Neuropsychopharmacol Biol Psychiatry 51: 105–112.
Ross HE, Young LJ (2009). Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol 30: 534–547.
Ryckmans T (2010). Modulation of the vasopressin system for the treatment of CNS diseases. Curr Opin Drug Discov Dev 13: 538–547.
Sala M, Braida D, Donzelli A, Martucci R, Busnelli M, Bulgheroni E et al (2013). Mice heterozygous for the oxytocin receptor gene show impaired social behaviour but not increased aggression or cognitive inflexibility: evidence of a selective haploinsufficiency gene effect. J Neuroendocrinol 25: 107–118.
Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V et al (2011). Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry 69: 875–882.
Saladin LK, Bruni JE (1993). The effects of intracerebroventricular versus intravenous administration of vasopressin on intracranial pressure in the rat. Neurol Res 15: 198–203.
Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H (2008). Negative BOLD with large increases in neuronal activity. Cereb Cortex 18: 1814–1827.
Sforazzini F, Schwarz AJ, Galbusera A, Bifone A, Gozzi A (2014). Distributed BOLD and CBV-weighted resting-state networks in the mouse brain. Neuroimage 87: 403–415.
Shalev I, Israel S, Uzefovsky F, Gritsenko I, Kaitz M, Ebstein RP (2011). Vasopressin needs an audience: neuropeptide elicited stress responses are contingent upon perceived social evaluative threats. Horm Behav 60: 121–127.
Shamay-Tsoory SG, Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biol Psychiatry 79: 194–202.
Sharma V, McNeill JH (2009). To scale or not to scale: the principles of dose extrapolation. Br J Pharmacol 157: 907–921.
Squillace M, Dodero L, Federici M, Migliarini S, Errico F, Napolitano F et al (2014). Dysfunctional dopaminergic neurotransmission in asocial BTBR mice. Transl Psychiatry 4: e427.
Sripada CS, Phan KL, Labuschagne I, Welsh R, Nathan PJ, Wood AG (2013). Oxytocin enhances resting-state connectivity between amygdala and medial frontal cortex. Int J Neuropsychopharmacol 16: 255–260.
Stoop R (2012). Neuromodulation by oxytocin and vasopressin. Neuron 76: 142–159.
Stoop R (2014). Neuromodulation by oxytocin and vasopressin in the central nervous system as a basis for their rapid behavioral effects. Curr Opin Neurobiol 29: 187–193.
Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T et al (2005). Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA 102: 16096–16101.
Thomas JS, Koh SH, Cooper GM (2007). Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth 98: 116–119.
US Food and Drug Administration (2005) Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Center for Drug Evaluation and Research (CDER). Federal Register Vol 70, No. 140: 05-14456.
Veening JG, Olivier B (2013). Intranasal administration of oxytocin: behavioral and clinical effects, a review. Neurosci Biobehav Rev 37: 1445–1465.
Walum H, Waldman ID, Young LJ (2016). Statistical and methodological considerations for the interpretation of intranasal oxytocin studies. Biol Psychiatry 79: 251–257.
Windle RJ, Shanks N, Lightman SL, Ingram CD (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats 1. Endocrinology 138: 2829–2834.
Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD (2004). Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity. J Neurosci 24: 2974–2982.
Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T et al (2009). Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci 29: 2259–2271.
Young LJ, Barrett CE (2015). Can oxytocin treat autism? Science 347: 825–826.
Young WS, Li J, Wersinger SR, Palkovits M (2006). The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience 143: 1031–1039.
Zaharchuk G, Mandeville JB, Bogdanov AA Jr., Weissleder R, Rosen BR, Marota JJ (1999). Cerebrovascular dynamics of autoregulation and hypoperfusion. An MRI study of CBF and changes in total and microvascular cerebral blood volume during hemorrhagic hypotension. Stroke 30: 2197–2204.
Zink CF, Meyer-Lindenberg A (2012). Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav 61: 400–409.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Galbusera, A., De Felice, A., Girardi, S. et al. Intranasal Oxytocin and Vasopressin Modulate Divergent Brainwide Functional Substrates. Neuropsychopharmacol 42, 1420–1434 (2017). https://doi.org/10.1038/npp.2016.283
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.283
This article is cited by
-
Oxytocinergic input from the paraventricular nucleus to the nucleus accumbens core modulates methamphetamine-conditioned place preference
Nature Communications (2025)
-
Early life oxytocin treatment improves thermo-sensory reactivity and maternal behavior in neonates lacking the autism-associated gene Magel2
Neuropsychopharmacology (2022)
-
Advances in the field of intranasal oxytocin research: lessons learned and future directions for clinical research
Molecular Psychiatry (2021)
-
In the nose or on the tongue? Contrasting motivational effects of oral and intranasal oxytocin on arousal and reward during social processing
Translational Psychiatry (2021)
-
Effects of route of administration on oxytocin-induced changes in regional cerebral blood flow in humans
Nature Communications (2020)