Abstract
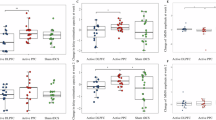
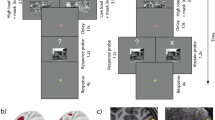
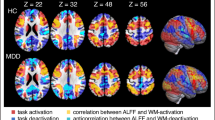
Working memory (WM) impairment, a core feature of schizophrenia, is often associated with aberrant dorsolateral prefrontal cortex (dlPFC) activation. Reduced resting-state connectivity within the frontoparietal control network (FPCN) has also been reported in schizophrenia. However, interpretation of WM-related dlPFC dysfunction has been limited by performance differences between patients and controls, and by uncertainty over the relevance of resting-state connectivity to network engagement during task. We contrasted brain activation in 40 schizophrenia patients and 40 controls during verbal WM performance, and evaluated underlying functional connectivity during rest and task. During correct trials, patients demonstrated normal FPCN activation, despite an inverse relationship between positive symptoms and activation. FPCN activation differed between the groups only during error trials (controls>patients). In contrast, controls demonstrated stronger deactivation of the ventromedial prefrontal cortex (vmPFC) during correct and error trials. Functional connectivity analysis indicated impaired resting-state FPCN connectivity in patients, but normal connectivity during task. However, patients showed abnormal connectivity among regions such as vmPFC, lateral orbitofrontal cortex, and parahippocampal gyrus (PHG) during both rest and task. During task, patients also exhibited altered thalamic connectivity to PHG and FPCN. Activation and connectivity patterns that were more characteristic of controls generally correlated with better performance. In summary, patients demonstrated normal FPCN activation when they remained on-task, and exhibited normal FPCN connectivity during WM, whereas vmPFC deactivation differences persisted regardless of WM performance. Our findings suggest that altered FPCN activation in patients reflects performance difference, and that limbic and thalamic dysfunction is critically involved in WM deficits in schizophrenia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Agam Y, Carey C, Barton JJ, Dyckman KA, Lee AK, Vangel M et al (2013). Network dynamics underlying speed-accuracy trade-offs in response to errors. PloS ONE 8: e73692.
Anticevic A, Repovs G, Krystal JH, Barch DM (2012). A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res 141: 8–14.
Anticevic A, Repovs G, Shulman GL, Barch DM (2010). When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage 49: 2638–2648.
Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL et al (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry 71: 109–118.
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA et al (2003). Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6: 750–757.
Buckner RL (2010). Human functional connectivity: new tools, unresolved questions. Proc Natl Acad Sci USA 107: 10769–10770.
Burock MA, Dale AM (2000). Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp 11: 249–260.
Carter CS, Perlstein W, Ganguli R, Brar J, Mintun M, Cohen JD (1998). Functional hypofrontality and working memory dysfunction in schizophrenia. Am J Psychiatry 155: 1285–1287.
Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S (2012). Anticorrelations in resting state networks without global signal regression. Neuroimage 59: 1420–1428.
Curtis CE, D'Esposito M (2003). Persistent activity in the prefrontal cortex during working memory. Trends Cognit Sci 7: 415–423.
D'Esposito M (2007). From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci 362: 761–772.
Dale AM, Buckner RL (1997). Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp 5: 329–340.
Eryilmaz H, Van De Ville D, Schwartz S, Vuilleumier P (2014). Lasting impact of regret and gratification on resting brain activity and its relation to depressive traits. J Neurosci 34: 7825–7835.
Fryer SL, Woods SW, Kiehl KA, Calhoun VD, Pearlson GD, Roach BJ et al (2013). Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front Psychiatry 4: 92.
Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD (2007). Aberrant "default mode" functional connectivity in schizophrenia. Am J Psychiatry 164: 450–457.
Goldin PR, McRae K, Ramel W, Gross JJ (2008). The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry 63: 577–586.
Hagler DJ Jr, Saygin AP, Sereno MI (2006). Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33: 1093–1103.
Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, Stein EA (2007). Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. J Neurosci 27: 3477–3489.
Heinzel S, Lorenz RC, Brockhaus WR, Wustenberg T, Kathmann N, Heinz A et al (2014). Working memory load-dependent brain response predicts behavioral training gains in older adults. J Neurosci 34: 1224–1233.
Jansma JM, Ramsey NF, van der Wee NJ, Kahn RS (2004). Working memory capacity in schizophrenia: a parametric fMRI study. Schizophr Res 68: 159–171.
Johnson MR, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA et al (2006). A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry 60: 11–21.
Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M et al (2007). The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophr Res 89: 191–197.
Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG et al (2009). Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp 30: 3795–3811.
Landin-Romero R, McKenna PJ, Salgado-Pineda P, Sarro S, Aguirre C, Sarri C et al (2014). Failure of deactivation in the default mode network: a trait marker for schizophrenia? Psychol Med 45: 1315–1325.
Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ et al (2015). A multimodal analysis of antipsychotic effects on brain structure and function in first-episode Schizophrenia. JAMA Psychiatry 72: 226–234.
Mang SC, Busza A, Reiterer S, Grodd W, Klose AU (2012). Thalamus segmentation based on the local diffusion direction: a group study. Magn Reson Med 67: 118–126.
Manoach DS (2003). Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res 60: 285–298.
Manoach DS, Gollub RL, Benson ES, Searl MM, Goff DC, Halpern E et al (2000). Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 48: 99–109.
Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E et al (1999). Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 45: 1128–1137.
Marenco S, Stein JL, Savostyanova AA, Sambataro F, Tan HY, Goldman AL et al (2012). Investigation of anatomical thalamo-cortical connectivity and FMRI activation in schizophrenia. Neuropsychopharmacology 37: 499–507.
Matsuo K, Chen SH, Liu CM, Liu CC, Hwang TJ, Hsieh MH et al (2013). Stable signatures of schizophrenia in the cortical-subcortical-cerebellar network using fMRI of verbal working memory. Schizophr Res 151: 133–140.
Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD (2009). Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. Plos ONE 4: e7911.
Menon V, Anagnoson RT, Mathalon DH, Glover GH, Pfefferbaum A (2001). Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms. Neuroimage 13: 433–446.
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66: 811–822.
Parnaudeau S, O'Neill PK, Bolkan SS, Ward RD, Abbas AI, Roth BL et al (2013). Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77: 1151–1162.
Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL et al (2008). Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain 131 (Pt 4): 971–986.
Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH et al (2009). Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull 35: 19–31.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59: 2142–2154.
Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA (2010). Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci USA 107: 10238–10243.
Schreiber S, Bernstein HG, Fendrich R, Stauch R, Ketzler B, Dobrowolny H et al (2011). Increased density of GAD65/67 immunoreactive neurons in the posterior subiculum and parahippocampal gyrus in treated patients with chronic schizophrenia. World J Biol Psychiatry 12: 57–65.
Seidman LJ, Thermenos HW, Poldrack RA, Peace NK, Koch JK, Faraone SV et al (2006). Altered brain activation in dorsolateral prefrontal cortex in adolescents and young adults at genetic risk for schizophrenia: an fMRI study of working memory. Schizophr Res 85: 58–72.
Sternberg S (1966). High-speed scanning in human memory. Science 153: 652–654.
Tanibuchi I, Goldman-Rakic PS (2003). Dissociation of spatial-, object-, and sound-coding neurons in the mediodorsal nucleus of the primate thalamus. J Neurophysiol 89: 1067–1077.
Van Snellenberg JX, Torres IJ, Thornton AE (2006). Functional neuroimaging of working memory in schizophrenia: task performance as a moderating variable. Neuropsychology 20: 497–510.
Walter H, Vasic N, Hose A, Spitzer M, Wolf RC (2007). Working memory dysfunction in schizophrenia compared to healthy controls and patients with depression: evidence from event-related fMRI. Neuroimage 35: 1551–1561.
Watanabe Y, Funahashi S (2012). Thalamic mediodorsal nucleus and working memory. Neurosci Biobehav Rev 36: 134–142.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW et al (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106: 1279–1284.
Wu G, Wang Y, Mwansisya TE, Pu W, Zhang H, Liu C et al (2014). Effective connectivity of the posterior cingulate and medial prefrontal cortices relates to working memory impairment in schizophrenic and bipolar patients. Schizophr Res 158: 85–90.
Yendiki A, Greve DN, Wallace S, Vangel M, Bockholt J, Mueller BA et al (2010). Multi-site characterization of an fMRI working memory paradigm: reliability of activation indices. Neuroimage 53: 119–131.
Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M et al (2011). The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106: 1125–1165.
Acknowledgements
We thank the study participants, and are grateful to Drs Daphne Holt and Isik Karahanoglu for helpful discussion relating to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Eryilmaz, H., Tanner, A., Ho, N. et al. Disrupted Working Memory Circuitry in Schizophrenia: Disentangling fMRI Markers of Core Pathology vs Other Aspects of Impaired Performance. Neuropsychopharmacol 41, 2411–2420 (2016). https://doi.org/10.1038/npp.2016.55
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.55
This article is cited by
-
Understanding translational research in schizophrenia: A novel insight into animal models
Molecular Biology Reports (2023)
-
Altered temporal, but intact spatial, features of transient network dynamics in psychosis
Molecular Psychiatry (2021)
-
Biochemical, physiological and clinical effects of l-methylfolate in schizophrenia: a randomized controlled trial
Molecular Psychiatry (2018)
-
A Longitudinal Functional Magnetic Resonance Imaging Study of Working Memory in Patients Following a Transient Ischemic Attack: A Preliminary Study
Neuroscience Bulletin (2018)
-
Using model systems to understand errant plasticity mechanisms in psychiatric disorders
Nature Neuroscience (2016)