Abstract
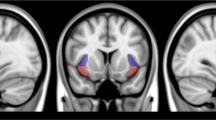
The ventral and dorsal striatum are critical substrates of reward processing and motivation and have been repeatedly linked to addictive disorders, including nicotine dependence. However, little is known about how functional connectivity between these and other brain regions is modulated by smoking withdrawal and may contribute to relapse vulnerability. In the present study, 37 smokers completed resting state fMRI scans during both satiated and 24-h abstinent conditions, prior to engaging in a 3-week quit attempt supported by contingency management. We examined the effects of abstinence condition and smoking outcome (lapse vs non-lapse) on whole-brain connectivity with ventral and dorsal striatum seed regions. Results indicated a significant condition by lapse outcome interaction for both right and left ventral striatum seeds. Robust abstinence-induced increases in connectivity with bilateral ventral striatum were observed across a network of regions implicated in addictive disorders, including insula, superior temporal gyrus, and anterior/mid-cingulate cortex among non-lapsers; the opposite pattern was observed for those who later lapsed. For dorsal striatum seeds, 24-h abstinence decreased connectivity across both groups with several regions, including medial prefrontal cortex, posterior cingulate cortex, hippocampus, and supplemental motor area. These findings suggest that modulation of striatal connectivity with the cingulo-insular network during early withdrawal may be associated with smoking cessation outcomes.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Addicott MA, Sweitzer MM, Froeliger B, Rose JE, McClernon FJ (2015). Increased functional connectivity in an insula-based network is associated with improved smoking cessation outcomes. Neuropsychopharmacology 40: 2648–2656.
Albert NB, Robertson EM, Miall RC (2009). The resting human brain and motor learning. Curr Biol 19: 1023–1027.
Buckner RL, Andrews-Hanna JR, Schacter DL (2008). The brain's default network: anatomy, function, and relevance to disease. Ann NY Acad Sci 1124: 1–38.
Buhler M, Vollstadt-Klein S, Kobiella A, Budde H, Reed LJ, Braus DF et al (2010). Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biol Psychiatry 67: 745–752.
Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES et al (2013). Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 365–376.
Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD (2010). Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage 52: 590–599.
Dallery J, Raiff BR, Grabinski MJ (2013). Internet-based contingency management to promote smoking cessation: a randomized controlled study. J Appl Behav Anal 46: 750–764.
David SP, Munafo MR, Johansen-Berg H, Smith SM, Rogers RD, Matthews PM et al (2005). Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: a functional magnetic resonance imaging study. Biol Psychiatry 58: 488–494.
Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z et al (2008). Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex 18: 2735–2747.
Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC et al (2006). A core system for the implementation of task sets. Neuron 50: 799–812.
Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y et al (2012). Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. NeuroImage 60: 252–262.
Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489.
Gianaros PJ, Manuck SB, Sheu LK, Kuan DC, Votruba-Drzal E, Craig AE et al (2011). Parental education predicts corticostriatal functionality in adulthood. Cereb Cortex 21: 896–910.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86: 1119–1127.
Hoffstaedter F, Grefkes C, Zilles K, Eickhoff SB (2013). The ‘what’ and ‘when’ of self-initiated movements. Cereb Cortex 23: 520–530.
Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B et al (2009). Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry 66: 431–441.
Hong LE, Hodgkinson CA, Yang Y, Sampath H, Ross TJ, Buchholz B et al (2010). A genetically modulated, intrinsic cingulate circuit supports human nicotine addiction. Proc Natl Acad Sci USA 107: 13509–13514.
Huang W, King JA, Ursprung WW, Zheng S, Zhang N, Kennedy DN et al (2014). The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain Behav 4: 408–417.
Hughes JR (2007). Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 9: 315–327.
Hughes JR, Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294.
Hughes JR, Shiffman S, Callas P, Zhang J (2003). A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control 12: 21–27.
Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend 125: 252–259.
Janes AC, Pizzagalli DA, Richardt S, de BFB, Chuzi S, Pachas G et al (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67: 722–729.
Kuhn S, Gallinat J (2011). Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci 33: 1318–1326.
Lerman C, Gu H, Loughead J, Ruparel K, Yang Y, Stein EA (2014). Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71: 523–530.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19: 1233–1239.
McClernon FJ, Kozink RV, Lutz AM, Rose JE (2009). 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl) 204: 25–35.
Middleton FA, Strick PL (2002). Basal-ganglia 'projections' to the prefrontal cortex of the primate. Cereb Cortex 12: 926–935.
Naqvi NH, Bechara A (2009). The hidden island of addiction: the insula. Trends Neurosci 32: 56–67.
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534.
Nestler EJ (2004). Molecular mechanisms of drug addiction. Neuropharmacology 47 (Suppl 1): 24–32.
Pierce RC, Vanderschuren LJ (2010). Kicking the habit: the neural basis of ingrained behaviors in cocaine addiction. Neurosci Biobehav Rev 35: 212–219.
Porter JN, Roy AK, Benson B, Carlisi C, Collins PF, Leibenluft E et al (2015). Age-related changes in the intrinsic functional connectivity of the human ventral vs. dorsal striatum from childhood to middle age. Dev Cogn Neurosci 11: 83–95.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682.
Reynolds SM, Zahm DS (2005). Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci 25: 11757–11767.
Roche DJ, Bujarski S, Moallem NR, Guzman I, Shapiro JR, Ray LA (2014). Predictors of smoking lapse in a human laboratory paradigm. Psychopharmacology (Berl) 231: 2889–2897.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011). The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12: 154–167.
Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA (2013). Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry 74: 538–546.
Sutherland MT, McHugh MJ, Pariyadath V, Stein EA (2012). Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage 62: 2281–2295.
Sweitzer MM, Geier CF, Denlinger R, Forbes EE, Raiff BR, Dallery J et al (2016). Blunted striatal response to monetary reward anticipation during smoking abstinence predicts lapse during a contingency-managed quit attempt. Psychopharmacology (Berl) 233: 751–760.
Sweitzer MM, Geier CF, Joel DL, McGurrin P, Denlinger RL, Forbes EE et al (2013). Dissociated effects of anticipating smoking versus monetary reward in the caudate as a function of smoking abstinence. Biol Psychiatry 76: 681–688.
Taylor KS, Seminowicz DA, Davis KD (2009). Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30: 2731–2745.
Tiffany ST, Conklin CA (2000). A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction 95 (Suppl 2): S145–S153.
Toll BA, Katulak NA, McKee SA (2006). Investigating the factor structure of the Questionnaire on Smoking Urges-Brief (QSU-Brief). AddictBehav 31: 1231–1239.
Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY et al (2014). Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob Res 16: 697–708.
Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F (2009). Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56 (Suppl 1): 3–8.
Wang K, Yang J, Zhang S, Wei D, Hao X, Tu S et al (2014). The neural mechanisms underlying the acute effect of cigarette smoking on chronic smokers. PLoS One 9: e102828.
Whitfield-Gabrieli S, Nieto-Castanon A (2012). Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2: 125–141.
Wilson SJ, Delgado MR, McKee SA, Grigson PS, MacLean RR, Nichols TT et al (2014). Weak ventral striatal responses to monetary outcomes predict an unwillingness to resist cigarette smoking. Cogn Affect Behav Neurosci 14: 1196–1207.
Wright CI, Groenewegen HJ (1996). Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience 73: 359–373.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Sweitzer, M., Geier, C., Addicott, M. et al. Smoking Abstinence-Induced Changes in Resting State Functional Connectivity with Ventral Striatum Predict Lapse During a Quit Attempt. Neuropsychopharmacol 41, 2521–2529 (2016). https://doi.org/10.1038/npp.2016.56
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.56
This article is cited by
-
Abnormal white matter tracts of insula in smokers
Brain Imaging and Behavior (2021)
-
The changes of brain functional networks in young adult smokers based on independent component analysis
Brain Imaging and Behavior (2021)
-
Intrinsic differences in insular circuits moderate the negative association between nicotine dependence and cingulate-striatal connectivity strength
Neuropsychopharmacology (2020)
-
The Impact of Combinations of Alcohol, Nicotine, and Cannabis on Dynamic Brain Connectivity
Neuropsychopharmacology (2018)
-
Striato-cortical tracts predict 12-h abstinence-induced lapse in smokers
Neuropsychopharmacology (2018)