Abstract
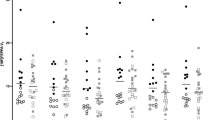
Several lines of evidence implicate microglial activation and abnormal immune response in the etiology of psychosis. Previous positron emission tomography (PET) neuroimaging studies of the translocator protein 18 kDa, TSPO, were limited by low affinity of the first-generation radioligand, low-resolution scanners, and small sample sizes. Moreover, there is a dearth of literature on microglial activation in individuals at clinical high risk (CHR) for psychosis. We used a novel second-generation TSPO radioligand, [18F]FEPPA, to examine whether microglial activation is elevated in the dorsolateral prefrontal cortex (DLPFC) and hippocampus of antipsychotic-naive CHR. Twenty-four CHR (antipsychotic-naive n=22) and 23 healthy volunteers (HV) completed a high resolution [18F]FEPPA PET scan and MRI. The PET data were analyzed using the validated two-tissue compartment model with arterial plasma input function with total volume of distribution (VT) as outcome measure. All analyses were controlled for the TSPO rs6971 polymorphism. We did not observe any significant differences in microglial activation, as indexed by [18F]FEPPA VT, between CHR and HV in either the DLPFC (F(1, 44)=0.41, p=0.52) or the hippocampus (F(1, 44)=2.78, p=0.10). Exploratory associations show that in CHR, [18F]FEPPA VT was positively correlated with apathy (DLPFC: r=0.55, p=0.008; hippocampus: r=0.52, p=0.013) and state anxiety (DLPFC: r=0.60, p=0.003; hippocampus: r=0.48, p=0.024). The lack of significant group differences in [18F]FEPPA VT suggests that microglial activation is not significantly elevated in the clinical high risk state that precedes psychosis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. (2015). Microglial activity in people at ultra high risk of psychosis and in schizophrenia: an [11C] PBR28 PET brain imaging study. Am J Psychiatry 173: 44–52.
Brown AS (2011). Further evidence of infectious insults in the pathogenesis and pathophysiology of schizophrenia. Am J Psychiatry 168: 764–766.
Cannon TD (2015). Microglial activation and the onset of psychosis. Am J Psychiatry 173: 3–4.
Collste K, Plavén-Sigray P, Fatouros-Bergman H, Victorsson P, Schain M, Forsberg A et al. (2017). Lower levels of the glial cell marker TSPO in drug-naive first-episode psychosis patients as measured using PET and [11C] PBR28. Mol Psychiatry 22: 850–856.
Coughlin J, Wang Y, Ambinder E, Ward R, Minn I, Vranesic M et al. (2016). In vivo markers of inflammatory response in recent-onset schizophrenia: a combined study using [ 11C] DPA-713 PET and analysis of CSF and plasma. Transl Psychiatry 6: e777.
Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC (2009). Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 50: 1801–1807.
Fernandes B, Steiner J, Bernstein H, Dodd S, Pasco J, Dean O et al. (2016). C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry 21: 554–564.
First M, Spitzer R, Gibbon M, Williams J (1995) Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P. Version 2.0. Biometric Research, New York State Psychiatric Institute: New York.
Fujita M, Imaizumi M, Zoghbi SS, Fujimura Y, Farris AG, Suhara T et al. (2008). Kinetic analysis in healthy humans of a novel positron emission tomography radioligand to image the peripheral benzodiazepine receptor, a potential biomarker for inflammation. Neuroimage 40: 43–52.
Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP et al. (2016). Imaging Microglial Activation in Untreated First-Episode Psychosis: A PET Study With [18F]FEPPA. Am J Psychiatry 174: 118–124.
Hines CS, Fujita M, Zoghbi SS, Kim JS, Quezado Z, Herscovitch P et al. (2013). Propofol decreases in vivo binding of 11C-PBR28 to translocator protein (18 kDa) in the human brain. J Nucl Med 54: 64–69.
Kenk M, Selvanathan T, Rao N, Suridjan I, Rusjan P, Remington G et al. (2015). Imaging neuroinflammation in gray and white matter in schizophrenia: an in-vivo PET study with [18F]-FEPPA. Schizophr Bull 41: 85–93.
Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB (2015). Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2: 258–270.
Kirkpatrick B, Miller BJ (2013). Inflammation and schizophrenia. Schizophr Bull 39: 1174–1179.
Ko JH, Koshimori Y, Mizrahi R, Rusjan P, Wilson AA, Lang AE et al. (2013). Voxel-based imaging of translocator protein 18 kDa (TSPO) in high-resolution PET. J Cereb Blood Flow Metab 33: 348–350.
Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL et al. (2013a). A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab 33: 53–58.
Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N et al. (2013b). In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain 136 (Pt 7): 2228–2238.
Meyer U, Schwarz MJ, Muller N (2011). Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther 132: 96–110.
Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K et al. (2002). Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry 159: 863–865.
Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I et al. (2012). Translocator protein (18 kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab 32: 968–972.
Muller-Gartner HW, Links JM, Prince JL, Bryan RN, McVeigh E, Leal JP et al. (1992). Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab 12: 571–583.
Narendran R, Frankle WG (2016). Comment on Analyses and Conclusions of ‘Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [11C] PBR28 PET Brain Imaging Study’. Am J Psychiatry 173: 536–537.
Notter T, Coughlin J, Gschwind T, Weber-Stadlbauer U, Wang Y, Kassiou M et al (2017). Translational evaluation of translocator protein as a marker of neuroinflammation in schizophrenia. Mol Psychiatry (in press).
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A et al. (2012). An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32: 1–5.
Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD et al. (2015). Towards a psychosis risk blood diagnostic for persons experiencing high-risk symptoms: preliminary results from the NAPLS project. Schizophr Bull 41: 419–428.
Perry VH, Cunningham C, Holmes C (2007). Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7: 161–167.
Randolph C (1998) Repeatable Battery for the Assessment of Neuropsychological Status. Psychological Corporation (Harcourt): San Antonio, TX.
Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A et al. (2001). Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry 58: 445–452.
Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F et al. (2006). An automated method for the extraction of regional data from PET images. Psychiatry Res 147: 79–89.
Rusjan PM, Wilson AA, Bloomfield PM, Vitcu I, Meyer JH, Houle S et al. (2011). Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab 31: 1807–1816.
Salim S, Chugh G, Asghar M (2012). Inflammation in anxiety. Adv Protein Chem Struct Biol 88: 1–25.
Sawada A, Niiyama Y, Ataka K, Nagaishi K, Yamakage M, Fujimiya M (2014). Suppression of bone marrow-derived microglia in the amygdala improves anxiety-like behavior induced by chronic partial sciatic nerve ligation in mice. Pain 155: 1762–1772.
Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–183.
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G et al. (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72: 268–275.
Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I et al. (2009). Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 460: 753–757.
Sommer IE, van Westrhenen R, Begemann MJ, de Witte LD, Leucht S, Kahn RS (2014). Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull 40: 181–191.
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al. (2009). Common variants conferring risk of schizophrenia. Nature 460: 744–747.
Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E et al. (2014). Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology 41: 23–32.
Suridjan I, Pollock BG, Verhoeff NP, Voineskos AN, Chow T, Rusjan PM et al. (2015). In-vivo imaging of grey and white matter neuroinflammation in Alzheimer's disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry 20: 1579–1587.
Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al. (2010). Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 13: 943–950.
Trepanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP (2016). Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry 21: 1009–1026.
van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. (2008). Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64: 820–822.
Venneti S, Wiley CA, Kofler J (2009). Imaging microglial activation during neuroinflammation and Alzheimer's disease. J Neuroimm Pharmacol 4: 227–243.
Vivash L, O'Brien TJ (2016). Imaging Microglial Activation with TSPO PET: Lighting Up Neurologic Diseases? J Nucl Med 57: 165–168.
Wilk CM, Gold JM, Humber K, Dickerson F, Fenton WS, Buchanan RW (2004). Brief cognitive assessment in schizophrenia: normative data for the Repeatable Battery for the Assessment of Neuropsychological Status. Schizophr Res 70: 175–186.
Wohleb ES, Powell ND, Godbout JP, Sheridan JF (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci 33: 13820–13833.
Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK et al. (2008). Increased binding of peripheral benzodiazepine receptor in Alzheimer's disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry 64: 835–841.
Zhang X, Paule MG, Newport GD, Liu F, Callicott R, Liu S et al. (2012). MicroPET/CT Imaging of [18F]-FEPPA in the nonhuman primate: a potential biomarker of pathogenic processes associated with anesthetic-induced neurotoxicity. ISRN Anesthesiol 2012.
Acknowledgements
We thank the excellent staff of the CAMH Research Imaging Centre and the FYPP clinic.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Hafizi, S., Da Silva, T., Gerritsen, C. et al. Imaging Microglial Activation in Individuals at Clinical High Risk for Psychosis: an In Vivo PET Study with [18F]FEPPA. Neuropsychopharmacol. 42, 2474–2481 (2017). https://doi.org/10.1038/npp.2017.111
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.111
This article is cited by
-
PET Imaging Unveils Neuroinflammatory Mechanisms in Psychiatric Disorders: From Microglial Activation to Therapeutic Innovation
Molecular Neurobiology (2025)
-
In vivo imaging translocator protein (TSPO) in autism spectrum disorder
Neuropsychopharmacology (2022)
-
The utility of PET imaging in the diagnosis and management of psychosis: a brief review
Clinical and Translational Imaging (2022)
-
Minocycline Ameliorates Chronic Unpredictable Mild Stress-Induced Neuroinflammation and Abnormal mPFC-HIPP Oscillations in Mice
Molecular Neurobiology (2022)
-
Applicability, potential and limitations of TSPO PET imaging as a clinical immunopsychiatry biomarker
European Journal of Nuclear Medicine and Molecular Imaging (2021)