Abstract
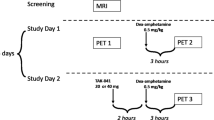
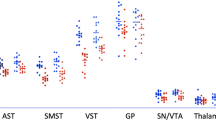
Individuals with drug use disorders seek drugs over other rewarding activities, and exhibit neurochemical deficits related to dopamine, which is involved in value-based learning and decision-making. Thus, a dopaminergic disturbance may underpin drug-biased choice in addiction. Classical drug-choice assessments, which offer drug-consumption opportunities, are inappropriate for addicted individuals seeking treatment or abstaining. Fifteen recently abstinent methamphetamine users and 15 healthy controls completed two laboratory paradigms of ‘simulated’ drug choice (choice for drug-related vs affectively pleasant, unpleasant, and neutral images), and underwent positron emission tomography measurements of dopamine D2-type receptor availability, indicated by binding potential (BPND) for [18F]fallypride. Thirteen of the methamphetamine users and 10 controls also underwent [11C]NNC112 PET scans to measure dopamine D1-type receptor availability. Group analyses showed that, compared with controls, methamphetamine users chose to view more methamphetamine-related images on one task, with a similar trend on the second task. Regression analyses showed that, on both tasks, the more methamphetamine users chose to view methamphetamine images, specifically vs pleasant images (the most frequently chosen images across all participants), the lower was their D2-type BPND in the lateral orbitofrontal cortex, an important region in value-based choice. No associations were observed with D2-type BPND in striatal regions, or with D1-type BPND in any region. These results identify a neurochemical correlate for a laboratory drug-seeking paradigm that can be administered to treatment-seeking and abstaining drug-addicted individuals. More broadly, these results refine the central hypothesis that dopamine-system deficits contribute to drug-biased decision-making in addiction, here showing a role for the orbitofrontal cortex.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abi-Dargham A, Martinez D, Mawlawi O, Simpson N, Hwang DR, Slifstein M et al (2000). Measurement of striatal and extrastriatal dopamine D1 receptor binding potential with [11C]NNC 112 in humans: validation and reproducibility. J Cereb Blood Flow Metab 20: 225–243.
Ahmed SH (2010). Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev 35: 172–184.
Ardekani BA, Braun M, Hutton BF, Kanno I, Iida H (1995). A fully automatic multimodality image registration algorithm. J Comput Assist Tomogr 19: 615–623.
Ashok AH, Mizuno Y, Volkow ND, Howes OD (2017). Association of stimulant use with dopaminergic alterations in users of cocaine, amphetamine, or methamphetamine: a systematic review and meta-analysis. JAMA Psychiatry 74: 511–519.
Ballard ME, Mandelkern MA, Monterosso JR, Hsu E, Robertson CL, Ishibashi K et al (2015). Low dopamine D2/D3 receptor availability is associated with steep discounting of delayed rewards in methamphetamine dependence. Int J Neuropsychopharmacol 18: pyu119.
Bartra O, McGuire JT, Kable JW (2013). The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76: 412–427.
Berridge KC, Kringelbach ML (2015). Pleasure systems in the brain. Neuron 86: 646–664.
Camerer C, Mobbs D (2017). Differences in behavior and brain activity during hypothetical and real choices. Trends Cogn Sci 21: 46–56.
Ekelund J, Slifstein M, Narendran R, Guillin O, Belani H, Guo NN et al (2007). In vivo DA D(1) receptor selectivity of NNC 112 and SCH 23390. Mol Imag Biol 9: 117–125.
First MB, Spitzer RL, Gibbon M, Williams J (1996) Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0). Biometrics Research Department, New York State Psychiatric Institute: New York, NY, USA.
Goldstein RZ, Volkow ND (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669.
Greenwald MK, Ledgerwood DM, Lundahl LH, Steinmiller CL (2014). Effect of experimental analogs of contingency management treatment on cocaine seeking behavior. Drug Alcohol Depend 139: 164–168.
Halldin C, Foged C, Chou YH, Karlsson P, Swahn CG, Sandell J et al (1998). Carbon-11-NNC 112: a radioligand for PET examination of striatal and neocortical D1-dopamine receptors. J Nuclear Mede 39: 2061–2068.
Hogarth L, Chase HW (2011). Parallel goal-directed and habitual control of human drug-seeking: implications for dependence vulnerability. J Exp Psychol Animal Behav Processes 37: 261–276.
Jenkinson M, Bannister P, Brady M, Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841.
Jenkinson M, Smith S (2001). A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156.
Kohno M, Ghahremani DG, Morales AM, Robertson CL, Ishibashi K, Morgan AT et al (2015). Risk-taking behavior: dopamine D2/D3 receptors, feedback, and frontolimbic activity. Cereb Cortex 25: 236–245.
Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED (2014). Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry 71: 812–820.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. Neuroimage 4: 153–158.
Lang PJ, Bradley MM, Cuthbert BN (2008) International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida: Gainsville, FL, USA.
Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR et al (2009). Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29: 14734–14740.
London ED, Ernst M, Grant S, Bonson K, Weinstein A (2000). Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex 10: 334–342.
London ED, Kohno M, Morales AM, Ballard ME (2015). Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res 1628: 174–185.
Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y et al (2004). Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 29: 1190–1202.
Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC et al (2011). Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry 168: 634–641.
Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A et al (2007). Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 164: 622–629.
Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR et al (2009). Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology 34: 1774–1782.
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR et al (2001). Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21: 1034–1057.
Moeller SJ, Beebe-Wang N, Woicik PA, Konova AB, Maloney T, Goldstein RZ (2013a). Choice to view cocaine images predicts concurrent and prospective drug use in cocaine addiction. Drug Alcohol Depend 130: 178–185.
Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F et al (2010). Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain 133: 1484–1493.
Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA et al (2009). Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry 66: 169–176.
Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N et al (2013b). Gene x abstinence effects on drug cue reactivity in addiction: multimodal evidence. J Neurosci 33: 10027–10036.
Moeller SJ, Stoops WW (2015). Cocaine choice procedures in animals, humans, and treatment-seekers: can we bridge the divide? Pharmacol Biochem Behav 138: 133–141.
Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M et al (2002). Brain imaging of 18 F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse 46: 170–188.
Mukherjee J, Constantinescu CC, Hoang AT, Jerjian T, Majji D, Pan ML (2015). Dopamine D3 receptor binding of (18)F-fallypride: Evaluation using in vitro and in vivo PET imaging studies. Synapse 69: 577–591.
Noonan MP, Mars RB, Rushworth MF (2011). Distinct roles of three frontal cortical areas in reward-guided behavior. J Neurosci 31: 14399–14412.
Padoa-Schioppa C (2011). Neurobiology of economic choice: a good-based model. Annu Rev Neurosci 34: 333–359.
Rudebeck PH, Mitz AR, Chacko RV, Murray EA (2013). Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80: 1519–1531.
Rudebeck PH, Murray EA (2014). The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron 84: 1143–1156.
Schmitt KC, Reith ME (2010). Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci 1187: 316–340.
Schuck NW, Cai MB, Wilson RC, Niv Y (2016). Human orbitofrontal cortex represents a cognitive map of state space. Neuron 91: 1402–1412.
Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA et al (2011). Dopaminergic modulation of risky decision-making. J Neurosci 31: 17460–17470.
Slifstein M, Kegeles LS, Gonzales R, Frankle WG, Xu X, Laruelle M et al (2007). [11C]NNC 112 selectivity for dopamine D1 and serotonin 5-HT(2 A) receptors: a PET study in healthy human subjects. J Cereb Blood Flow Metab 27: 1733–1741.
Stopper CM, Khayambashi S, Floresco SB (2013). Receptor-specific modulation of risk-based decision making by nucleus accumbens dopamine. Neuropsychopharmacology 38: 715–728.
Surti S, Kuhn A, Werner ME, Perkins AE, Kolthammer J, Karp JS (2007). Performance of Philips Gemini TF PET/CT scanner with special consideration for its time-of-flight imaging capabilities. J Nuclear Med 48: 471–480.
Volkow ND, Fowler JS (2000). Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10: 318–325.
Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D et al (2012). Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry 17: 918–925.
Wu Y, Carson RE (2002). Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22: 1440–1452.
Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K (2016). Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 531: 642–646.
Acknowledgements
We thank Maritza Johnson for assistance with this study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Moeller, S., Okita, K., Robertson, C. et al. Low Striatal Dopamine D2-type Receptor Availability is Linked to Simulated Drug Choice in Methamphetamine Users. Neuropsychopharmacol. 43, 751–760 (2018). https://doi.org/10.1038/npp.2017.138
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.138
This article is cited by
-
Is addiction a brain disease? A plea for agnosticism and heterogeneity
Psychopharmacology (2022)
-
No effect of attentional bias modification training in methamphetamine users receiving residential treatment
Psychopharmacology (2019)
-
Genome-wide transcriptional profiling of central amygdala and orbitofrontal cortex during incubation of methamphetamine craving
Neuropsychopharmacology (2018)