Abstract
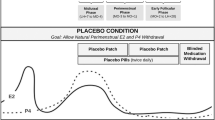
The mechanisms linking ovarian hormones to negative affect are poorly characterized, but important clues may come from the examination of the brain’s intrinsic organization. Here, we studied the effects of both the menstrual cycle and oral contraceptives (OCs) on amygdala and salience network resting-state functional connectivity using a double-blind, randomized, and placebo-controlled design. Hormone levels, depressive symptoms, and resting-state functional connectivity were measured in 35 healthy women (24.9±4.2 years) who had previously experienced OC-related negative affect. All participants were examined in the follicular phase of a baseline cycle and in the third week of the subsequent cycle during treatment with either a combined OC (30 μg ethinyl estradiol/0.15 mg levonorgestrel) or placebo. The latter time point targeted the midluteal phase in placebo users and steady-state ethinyl estradiol and levonorgestrel concentrations in OC users. Amygdala and salience network connectivity generally increased with both higher endogenous and synthetic hormone levels, although amygdala–parietal cortical connectivity decreased in OC users. When in the luteal phase, the naturally cycling placebo users demonstrated higher connectivity in both networks compared with the women receiving OCs. Our results support a causal link between the exogenous administration of synthetic hormones and amygdala and salience network connectivity. Furthermore, they suggest a similar, potentially stronger, association between the natural hormonal variations across the menstrual cycle and intrinsic network connectivity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I et al (2015). Progesterone mediates brain functional connectivity changes during the menstrual cycle–a pilot resting-state MRI study. Front Neurosci 9: 44.
Brakowski J, Spinelli S, Dörig N, Bosch OG, Manoliu A, Holtforth MG et al (2017). Resting state brain network function in major depression – depression symptomatology, antidepressant treatment effects, future research. J Psychiatr Res 92: 147–159.
Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE et al (2008). Progesterone receptors: form and function in brain. Front Neuroendocrinol 29: 313–339.
Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E (2015). Perimenopause as a neurological transition state. Nat Rev Endocrinol 11: 393–405.
Craddock RC, Jbabdi S, Yan C-G, Vogelstein JT, Castellanos FX, Di Martino A et al (2013). Imaging human connectomes at the macroscale. Nat Methods 10: 524–539.
De Bondt T, Smeets D, Pullens P, Van Hecke W, Jacquemyn Y, Parizel PM (2015). Stability of resting state networks in the female brain during hormonal changes and their relation to premenstrual symptoms. Brain Res 1624: 275–285.
Engman J, Linnman C, Van Dijk KRA, Milad MR (2016). Amygdala subnuclei resting-state functional connectivity sex and estrogen differences. Psychoneuroendocrinology 63: 34–42.
Fisher PM, Larsen CB, Beliveau V, Henningsson S, Pinborg A, Holst KK et al (2017). Pharmacologically induced sex hormone fluctuation effects on resting-state functional connectivity in a risk model for depression: a randomized trial. Neuropsychopharmacology 42: 446–453.
Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF et al (2014). Emotion regulation: quantitative meta-analysis of functional activation and deactivation. Neurosci Biobehav Rev 45: 202–211.
Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E et al (2014). The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage 95: 193–207.
Gingnell M, Bannbers E, Engman J, Frick A, Moby L, Wikström J et al (2016). The effect of combined hormonal contraceptives use on brain reactivity during response inhibition. Eur J Contracept Reprod Health Care 21: 150–157.
Gingnell M, Engman J, Frick A, Moby L, Wikström J, Fredrikson M et al (2013). Oral contraceptive use changes brain activity and mood in women with previous negative affect on the pill-A double-blinded, placebo-controlled randomized trial of a levonorgestrel-containing combined oral contraceptive. Psychoneuroendocrinology 38: 1133–1144.
Gordon EM, Breeden AL, Bean SE, Vaidya CJ (2014). Working memory-related changes in functional connectivity persist beyond task disengagement. Hum Brain Mapp 35: 1004–1017.
Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH et al (2012). One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 61: 1471–1483.
Hapgood JP, Africander D, Louw R, Ray RM, Rohwer JM (2014). Potency of progestogens used in hormonal therapy: toward understanding differential actions. J Steroid Biochem Mol Biol 142: 39–47.
Henningsson S, Madsen KH, Pinborg A, Heede M, Knudsen GM, Siebner HR et al (2015). Role of emotional processing in depressive responses to sex-hormone manipulation: a pharmacological fMRI study. Transl Psychiatry 5: e688.
Hjelmervik H, Hausmann M, Osnes B, Westerhausen R, Specht K (2014). Resting states are resting traits - an fMRI study of sex differences and menstrual cycle effects in resting state cognitive control networks. PLoS ONE 9: e103492.
Jenkinson M, Bannister P, Brady M, Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841.
Koziol LF, Budding DE, Chidekel D (2011). Sensory integration, sensory processing, and sensory modulation disorders: putative functional neuroanatomic underpinnings. Cerebellum 10: 770–792.
Lisofsky N, Mårtensson J, Eckert A, Lindenberger U, Gallinat J, Kühn S (2015). Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage 118: 154–162.
Lundin C, Danielsson KG, Bixo M, Moby L, Bengtsdotter H, Jawad I et al (2017). Combined oral contraceptive use is associated with both improvement and worsening of mood in the different phases of the treatment cycle-a double-blind, placebo-controlled randomized trial. Psychoneuroendocrinology 76: 135–143.
Mahfouz A, Lelieveldt BPF, Grefhorst A, van Weert LTCM, Mol IM, Sips HCM et al (2016). Genome-wide coexpression of steroid receptors in the mouse brain: identifying signaling pathways and functionally coordinated regions. Proc Natl Acad Sci USA 113: 2738–2743.
Menon V. Salience Network. In: Toga AW (ed). Brain Mapping: An Encyclopedic Reference, vol 2. Elsevier/Academic Press: Amsterdam, 2015, pp 597–611..
Montgomery SA, Asberg M (1979). A new depression scale designed to be sensitive to change. Br J Psychiatry 134: 382–389.
Moses EL, Drevets WC, Smith G, Mathis CA, Kalro BN, Butters MA et al (2000). Effects of estradiol and progesterone administration on human serotonin 2A receptor binding: A PET study. Biol Psychiatry 48: 854–860.
Nevatte T, O’Brien PMS, Bäckström T, Brown C, Dennerstein L, Endicott J et al (2013). ISPMD consensus on the management of premenstrual disorders. Arch Womens Ment Health 16: 279–291.
Nolte J (2009) The Human Brain: An Introduction to its Functional Anatomy. Mosby: Philadelphia, PA.
Olivier B (2015). Serotonin: a never-ending story. Eur J Pharmacol 753: 2–18.
Ottowitz WE, Derro D, Dougherty DD, Lindquist MA, Fischman AJ, Hall JE (2008a). FDG-PET analysis of amygdalar-cortical network covariance during pre- versus post-menopausal estrogen levels: potential relevance to resting state networks, mood, and cognition. Neuroendocrinol Lett 29: 467–474.
Ottowitz WE, Siedlecki KL, Lindquist MA, Dougherty DD, Fischman AJ, Hall JE (2008b). Evaluation of prefrontal-hippocampal effective connectivity following 24 hours of estrogen infusion: an FDG-PET study. Psychoneuroendocrinology 33: 1419–1425.
Parsons B, Rainbow TC, MacLusky NJ, McEwen BS (1982). Progestin receptor levels in rat hypothalamic and limbic nuclei. J Neurosci 2: 1446–1452.
Peper JS, Heuvel MP, van den, Mandl RCW, Pol HEH, van Honk J (2011). Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology 36: 1101–1113.
Petersen N, Cahill L (2015). Amygdala reactivity to negative stimuli is influenced by oral contraceptive use. Soc Cogn Affect Neurosci 10: 1266–1272.
Petersen N, Kilpatrick LA, Goharzad A, Cahill L (2014). Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. Neuroimage 90: 24–32.
Pletzer B, Crone JS, Kronbichler M, Kerschbaum H (2016). Menstrual cycle and hormonal contraceptive-dependent changes in intrinsic connectivity of resting-state brain networks correspond to behavioral changes due to hormonal status. Brain Connect 6: 572–585.
Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K et al (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626.
Sacher J, Rekkas PV, Wilson AA, Houle S, Romano L, Hamidi J et al (2015). Relationship of monoamine oxidase-a distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology 40: 429–435.
Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T et al (2012). Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage 61: 1213–1225.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59: 22–33.
Skovlund C, Mørch L, Kessing L, Lidegaard Ø (2016). Association of hormonal contraception with depression. JAMA Psychiatry 73: 1154–1162.
Sundström Poromaa I, Gingnell M (2014). Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front Neurosci 8: 380.
Sundström Poromaa I, Segebladh B (2012). Adverse mood symptoms with oral contraceptives. Acta Obstet Gynecol Scand 91: 420–427.
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N et al (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15: 273–289.
Vaidya CJ, Gordon EM (2013). Phenotypic variability in resting-state functional connectivity: current status. Brain Connect 3: 99–120.
Yan C-G, Zang Y-F (2010). DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4: 13.
Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A et al (2011). Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 70: 920–927.
Zhang Y, Gong J, Xie C, Ye EM, Jin X, Song H et al (2015). Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: evidence from resting state fMRI. Neuroscience 284: 998–1010.
Acknowledgements
The amygdala resting-state functional connectivity data were presented in a short oral presentation at the 32nd Annual Scientific Meeting of the European Society for Magnetic Resonance in Medicine and Biology, Edinburgh, UK, and the salience network resting-state functional connectivity data as a poster at the 22nd Annual Meeting of the Organization for Human Brain Mapping, Geneva, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Engman, J., Sundström Poromaa, I., Moby, L. et al. Hormonal Cycle and Contraceptive Effects on Amygdala and Salience Resting-State Networks in Women with Previous Affective Side Effects on the Pill. Neuropsychopharmacol. 43, 555–563 (2018). https://doi.org/10.1038/npp.2017.157
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.157
This article is cited by
-
The interplay of central insulin and menstrual cycle on functional brain networks and neural food cue reactivity in women
Communications Biology (2026)
-
Whole-brain dynamics across the menstrual cycle: the role of hormonal fluctuations and age in healthy women
npj Women's Health (2024)
-
Effects of exogenous oxytocin and estradiol on resting-state functional connectivity in women and men
Scientific Reports (2023)
-
Triple network model of brain connectivity changes related to adverse mood effects in an oral contraceptive placebo-controlled trial
Translational Psychiatry (2023)
-
Psychobiological risk factors for suicidal thoughts and behaviors in adolescence: a consideration of the role of puberty
Molecular Psychiatry (2022)