Abstract
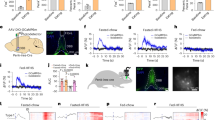
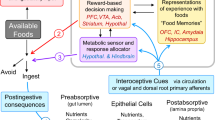
Long-chain fatty acids (FAs) act centrally to decrease food intake and hepatic glucose production and alter hypothalamic neuronal activity in a manner that depends on FA type and cellular transport proteins. However, it is not known whether FAs are sensed by ventral tegmental area (VTA) dopamine (DA) neurons to control food-motivated behavior and DA neurotransmission. We investigated the impact of the monounsaturated FA oleate in the VTA on feeding, locomotion, food reward, and DA neuronal activity and DA neuron expression of FA-handling proteins and FA uptake. A single intra-VTA injection of oleate, but not of the saturated FA palmitate, decreased food intake and increased locomotor activity. Furthermore, intra-VTA oleate blunted the rewarding effects of high-fat/sugar food in an operant task and inhibited DA neuronal firing. Using sorted DA neuron preparations from TH-eGFP mice we found that DA neurons express FA transporter and binding proteins, and are capable of intracellular transport of long-chain FA. Finally, we demonstrate that a transporter blocker attenuates FA uptake into DA neurons and blocks the effects of intra-VTA oleate to decrease food-seeking and DA neuronal activity. Together, these results suggest that DA neurons detect FA and that oleate has actions in the VTA to suppress DA neuronal activity and food seeking following cellular incorporation. These findings highlight the capacity of DA neurons to act as metabolic sensors by responding not only to hormones but also to FA nutrient signals to modulate food-directed behavior.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S et al (2009). Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest 119: 2577–2589.
Bixel MG, Hamprecht B (1995). Generation of ketone bodies from leucine by cultured astroglial cells. J Neurochem 65: 2450–2461.
Bouyakdan K, Taib B, Budry L, Zhao S, Rodaros D, Neess D et al (2015). A novel role for central ACBP/DBI as a regulator of long-chain fatty acid metabolism in astrocytes. J Neurochem 133: 253–265.
Cansell C, Castel J, Denis RG, Rouch C, Delbes AS, Martinez S et al (2014). Dietary triglycerides act on mesolimbic structures to regulate the rewarding and motivational aspects of feeding. Mol Psychiatry 19: 1095–1105.
Carten JD, Bradford MK, Farber SA (2011). Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish. Dev Biol 360: 276–285.
Chabowski A, Żendzian-Piotrowska M, Konstantynowicz K, Pankiewicz W, Mikłosz A, Łukaszuk B et al (2013). Fatty acid transporters involved in the palmitate and oleate induced insulin resistance in primary rat hepatocytes. Acta Physiol 207: 346–357.
Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O (2005). Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet 14: 1709–1725.
Contreras CM, Rodriguez-Landa JF, Garcia-Rios RI, Cueto-Escobedo J, Guillen-Ruiz G, Bernal-Morales B (2014). Myristic acid produces anxiolytic-like effects in Wistar rats in the elevated plus maze. BioMed Res Int 2014: 492141.
Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ et al (2008). Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci 122: 1257–1263.
Décarie-Spain L, Hryhorczuk C, Fulton S (2016). Dopamine signalling adaptations by prolonged high-fat feeding. Curr Opin Behav Sci 9: 136–143.
Fasano C, Thibault D, Trudeau LE (2008). Culture of postnatal mesencephalic dopamine neurons on an astrocyte monolayer. Curr Protoc Neurosci Chapter 3: Unit 3.21.
Fernandes MF, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T et al (2015). Leptin suppresses the rewarding effects of running via STAT3 signaling in dopamine neurons. Cell Metab 22: 741–749.
Fortin SM, Chartoff EH, Roitman MF (2016). The aversive agent lithium chloride suppresses phasic dopamine release through central GLP-1 receptors. Neuropsychopharmacology 41: 906–915.
Fulton S (2010). Appetite and reward. Front Neuroendocrinol 31: 85–103.
Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN et al (2006). Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51: 811–822.
Fulton S, Thibault D, Mendez JA, Lahaie N, Tirotta E, Borrelli E et al (2011). Contribution of Kv1.2 voltage-gated potassium channel to D2 autoreceptor regulation of axonal dopamine overflow. J Biol Chem 286: 9360–9372.
Gobbi M, Mennini T, Dalla Valle F, Cervo L, Salmona M, Diomede L (1999). Oleamide-mediated sleep induction does not depend on perturbation of membrane homeoviscosity. FEBS Lett 463: 281–284.
Greene JG, Dingledine R, Greenamyre JT (2005). Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis 18: 19–31.
Grimm J, Mueller A, Hefti F, Rosenthal A (2004). Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci USA 101: 13891–13896.
Guzman M, Blazquez C (2001). Is there an astrocyte-neuron ketone body shuttle? Trends Endocrinol Metab 12: 169–173.
Hryhorczuk C, Florea M, Rodaros D, Poirier I, Daneault C, Des Rosiers C et al (2015). Dampened mesolimbic dopamine function and signaling by saturated but not monounsaturated dietary lipids. Neuropsychopharmacology 41: 811–821.
Jo YH, Su Y, Gutierrez-Juarez R, Chua S Jr. (2009). Oleic acid directly regulates POMC neuron excitability in the hypothalamus. J Neurophysiol 101: 2305–2316.
Johnson PM, Kenny PJ (2010). Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci 13: 635–641.
Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K et al (2010). Increased brain fatty acid uptake in metabolic syndrome. Diabetes 59: 2171–2177.
Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ et al (2011). Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab 14: 173–183.
Kleinridders A, Schenten D, Konner AC, Belgardt BF, Mauer J, Okamura T et al (2009). MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 10: 249–259.
Labouebe G, Liu S, Dias C, Zou H, Wong JC, Karunakaran S et al (2013). Insulin induces long-term depression of ventral tegmental area dopamine neurons via endocannabinoids. Nat Neurosci 16: 300–308.
Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L et al (2005). Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11: 320–327.
Le Foll C, Dunn-Meynell A, Musatov S, Magnan C, Levin BE (2013). FAT/CD36: a major regulator of neuronal fatty acid sensing and energy homeostasis in rats and mice. Diabetes 62: 2709–2716.
Le Foll C, Dunn-Meynell AA, Miziorko HM, Levin BE (2014). Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes 63: 1259–1269.
Le Foll C, Irani BG, Magnan C, Dunn-Meynell AA, Levin BE (2009). Characteristics and mechanisms of hypothalamic neuronal fatty acid sensing. Am J Physiol Regul Integr Comp Physiol 297: R655–R664.
Liu RZ, Mita R, Beaulieu M, Gao Z, Godbout R (2010). Fatty acid binding proteins in brain development and disease. Int J Dev Biol 54: 1229–1239.
Liu S, Borgland SL (2015). Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience 289: 19–42.
Ma W, Berg J, Yellen G (2007). Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci 27: 3618–3625.
Matsushita N, Okada H, Yasoshima Y, Takahashi K, Kiuchi K, Kobayashi K (2002). Dynamics of tyrosine hydroxylase promoter activity during midbrain dopaminergic neuron development. J Neurochem 82: 295–304.
Medina JM, Tabernero A (2002). Astrocyte-synthesized oleic acid behaves as a neurotrophic factor for neurons. J Physiol Paris 96: 265–271.
Mendez JA, Bourque M-J, Bo GD, Bourdeau ML, Danik M, Williams S et al (2008). Developmental and target-dependent regulation of vesicular glutamate transporter expression by dopamine neurons. J Neurosci 28: 6309–6318.
Mitchell RW, Hatch GM (2011a). Fatty acid transport into the brain: of fatty acid fables and lipid tails. Prostaglandins Leukot Essent Fatty Acids 85: 293–302.
Mitchell RW, On NH, Del Bigio MR, Miller DW, Hatch GM (2011b). Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J Neurochem 117: 735–746.
Morales M, Margolis EB (2017). Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18: 73–85.
Moulle VS, Cansell C, Luquet S, Cruciani-Guglielmacci C (2012). The multiple roles of fatty acid handling proteins in brain. Front Physiol 3: 385.
Moulle VS, Le Foll C, Philippe E, Kassis N, Rouch C, Marsollier N et al (2013). Fatty acid transporter CD36 mediates hypothalamic effect of fatty acids on food intake in rats. PLoS ONE 8: e74021.
Obici S, Feng Z, Arduini A, Conti R, Rossetti L (2003). Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9: 756–761.
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L (2002). Central administration of oleic acid inhibits glucose production and food intake. Diabetes 51: 271–275.
Oomura Y, Nakamura T, Sugimori M, Yamada Y (1975). Effect of free fatty acid on the rat lateral hypothalamic neurons. Physiol Behav 14: 483–486.
Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: Cambridge, MA, 1998..
Picard A, Moulle VS, Le Foll C, Cansell C, Veret J, Coant N et al (2014a). Physiological and pathophysiological implications of lipid sensing in the brain. Diabetes Obes Metab 16 (Suppl 1): 49–55.
Picard A, Rouch C, Kassis N, Moulle VS, Croizier S, Denis RG et al (2014b). Hippocampal lipoprotein lipase regulates energy balance in rodents. Mol Metab 3: 167–176.
Ross RA, Rossetti L, Lam TK, Schwartz GJ (2010). Differential effects of hypothalamic long-chain fatty acid infusions on suppression of hepatic glucose production. Am J Physiol Endocrinol Metab 299: E633–E639.
Schwinkendorf DR, Tsatsos NG, Gosnell BA, Mashek DG (2011). Effects of central administration of distinct fatty acids on hypothalamic neuropeptide expression and energy metabolism. Int J Obes (Lond) 35: 336–344.
Sharma S, Hryhorczuk C, Fulton S (2012). Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J Vis Exp 63: e3754.
Sheng Z, Santiago AM, Thomas MP, Routh VH (2014). Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol Cell Neurosci 62: 30–41.
Shioda N, Yabuki Y, Kobayashi Y, Onozato M, Owada Y, Fukunaga K (2014). FABP3 protein promotes alpha-synuclein oligomerization associated with 1-methyl-1,2,3,6-tetrahydropiridine-induced neurotoxicity. J Biol Chem 289: 18957–18965.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008). Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 117: 924–935.
Taib B, Bouyakdan K, Hryhorczuk C, Rodaros D, Fulton S, Alquier T (2013). Glucose regulates hypothalamic long-chain fatty acid metabolism via AMP-activated kinase (AMPK) in neurons and astrocytes. J Biol Chem 288: 37216–37229.
Tan MC, Matsuoka S, Ano H, Ishida H, Hirose M, Sato F et al (2014). Interaction kinetics of liposome-incorporated unsaturated fatty acids with fatty acid-binding protein 3 by surface plasmon resonance. Bioorg Med Chem 22: 1804–1808.
Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limon P, Ren X et al (2013). A gut lipid messenger links excess dietary fat to dopamine deficiency. Science (New York, NY) 341: 800–802.
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al (2001). Brain dopamine and obesity. Lancet 357: 354–357.
Wang R, Cruciani-Guglielmacci C, Migrenne S, Magnan C, Cotero VE, Routh VH (2006). Effects of oleic acid on distinct populations of neurons in the hypothalamic arcuate nucleus are dependent on extracellular glucose levels. J Neurophysiol 95: 1491–1498.
Acknowledgements
We thank Marie-Josée Bourque for neuron cultures and help with cell sorting, and Demetra Rodaros and Anna Kristyna Franco Flores for helping with intra-VTA surgeries and operant responding experiments. This work was supported by a CIHR grant (MOP123280) and New Investigator salary award to SF, by a CIHR grant (MOP115042) and Fonds de Recherche Québec-Santé salary award to TA, by CIHR (MOP106556) and Brain Canada/Krembil Foundation grants to L-ET, American Heart Association grants to VHR (14GRNT20380639 and 1RO1DK103676), and doctoral scholarships from the Montreal Diabetes Research Center/Université de Montréal and CMDO/Novo Nordisk to CH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Hryhorczuk, C., Sheng, Z., Décarie-Spain, L. et al. Oleic Acid in the Ventral Tegmental Area Inhibits Feeding, Food Reward, and Dopamine Tone. Neuropsychopharmacol. 43, 607–616 (2018). https://doi.org/10.1038/npp.2017.203
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.203
This article is cited by
-
Receptor and metabolic insights on the ability of caffeine to prevent alcohol-induced stimulation of mesolimbic dopamine transmission
Translational Psychiatry (2024)
-
The Neuroinflammatory and Neurotoxic Potential of Palmitic Acid Is Mitigated by Oleic Acid in Microglial Cells and Microglial-Neuronal Co-cultures
Molecular Neurobiology (2021)
-
Cocaine- and amphetamine-regulated transcript peptide- and dopamine-containing systems interact in the ventral tegmental area of the zebra finch, Taeniopygia guttata, during dynamic changes in energy status
Brain Structure and Function (2021)
-
Lipid signalling in the mesolimbic dopamine pathway
Neuropsychopharmacology (2019)