Abstract
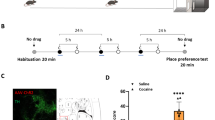
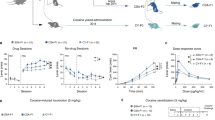
Mechanistic target of rapamycin (mTOR) regulates long-term synaptic plasticity, learning, and memory by controlling dendritic protein synthesis. The mTOR inhibitor rapamycin has been shown to attenuate the behavioral effects of drugs of abuse, including cocaine. Using viral vectors to selectively delete mTOR in the ventral tegmental area (VTA) in adult male mTORloxP/loxP mice, we investigated the role of mTOR in regulating neuronal morphology, basal synaptic transmission, dopamine dynamics, and cocaine-induced synaptic plasticity and rewarding effects. We find that targeted deletion of mTOR in the VTA had no significant effects on soma size and dendritic morphology of VTA neurons but significantly decreased dopamine release and reuptake in the nucleus accumbens (NAc) shell, a major target region. Western blot analysis revealed that mTOR deletion led to decreases in phosphorylated tyrosine hydroxylase (pTH-Ser40) levels in the VTA and dopamine transporter expression in the NAc. mTOR deletion had no significant effects on basal excitatory transmission in VTA dopamine neurons but caused an increase in GABAergic inhibition because of an increase in VTA GABAergic neuron firing. Furthermore, mTOR deletion attenuated conditioned place preference to cocaine and cocaine-induced potentiation of excitation and reduction of GABAergic inhibition in VTA dopamine neurons. Taken together, these results suggest that loss of mTOR in the VTA shifts the balance of excitatory and inhibitory synaptic transmission and decreases dopamine release and reuptake in the NAc. In addition, VTA mTOR signaling regulates cocaine-cue associative learning and cocaine-induced synaptic plasticity in VTA dopamine neurons.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM (2008). Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci 28: 8821–8831.
Argilli E, Sibley DR, Malenka RC, England PM, Bonci A (2008). Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci 28: 9092–9100.
Bailey J, Ma D, Szumlinski KK (2012). Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol 17: 248–258.
Barak S, Liu F, Ben Hamida S, Yowell QV, Neasta J, Kharazia V et al (2013). Disruption of alcohol-related memories by mTORC1 inhibition prevents relapse. Nat Neurosci 16: 1111–1117.
Bateup HS, Johnson CA, Denefrio CL, Saulnier JL, Kornacker K, Sabatini BL (2013). Excitatory/inhibitory synaptic imbalance leads to hippocampal hyperexcitability in mouse models of tuberous sclerosis. Neuron 78: 510–522.
Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D (2016). The first alcohol drink triggers mTORC1-dependent synaptic plasticity in nucleus accumbens dopamine D1 receptor neurons. J Neurosci 36: 701–713.
Bocklisch C, Pascoli V, Wong JC, House DR, Yvon C, de Roo M et al (2013). Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341: 1521–1525.
Borgland SL, Malenka RC, Bonci A (2004). Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci 24: 7482–7490.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493: 532–536.
Chow DK, Groszer M, Pribadi M, Machniki M, Carmichael ST, Liu X et al (2009). Laminar and compartmental regulation of dendritic growth in mature cortex. Nat Neurosci 12: 116–118.
Christian DT, Wang X, Chen EL, Sehgal LK, Ghassemlou MN, Miao JJ et al (2017). Dynamic alterations of rat nucleus accumbens dendritic spines over 2 months of abstinence from extended-access cocaine self-administration. Neuropsychopharmacology 42: 748–756.
Costa-Mattioli M, Monteggia LM (2013). mTOR complexes in neurodevelopmental and neuropsychiatric disorders. Nat Neurosci 16: 1537–1543.
Dadalko OI, Siuta M, Poe A, Erreger K, Matthies HJ, Niswender K et al (2015). mTORC2/rictor signaling disrupts dopamine-dependent behaviors via defects in striatal dopamine neurotransmission. J Neurosci 35: 8843–8854.
Dayas CV, Smith DW, Dunkley PR (2012). An emerging role for the mammalian target of rapamycin in "pathological" protein translation: relevance to cocaine addiction. Front Pharmacol 3: 13.
Ding S, Matta SG, Zhou FM (2011). Kv3-like potassium channels are required for sustained high-frequency firing in basal ganglia output neurons. J Neurophysiol 105: 554–570.
Dong Y, Nestler EJ (2014). The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 35: 374–383.
Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T et al (2004). Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(-/-) mice. Proc Natl Acad Sci USA 101: 14282–14287.
Dumitriu D, Rodriguez A, Morrison JH (2011). High-throughput, detailed, cell-specific neuroanatomy of dendritic spines using microinjection and confocal microscopy. Nat Protoc 6: 1391–1411.
Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW (2004). Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 91: 1025–1043.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG (1996). Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379: 606–612.
Hernandez D, Torres CA, Setlik W, Cebrian C, Mosharov EV, Tang G et al (2012). Regulation of presynaptic neurotransmission by macroautophagy. Neuron 74: 277–284.
Hoeffer CA, Klann E (2010). mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75.
Huang W, Zhu PJ, Zhang S, Zhou H, Stoica L, Galiano M et al (2013). mTORC2 controls actin polymerization required for consolidation of long-term memory. Nat Neurosci 16: 441–448.
Huber KM, Klann E, Costa-Mattioli M, Zukin RS (2015). Dysregulation of mammalian target of rapamycin signaling in mouse models of autism. J Neurosci 35: 13836–13842.
James MH, Quinn RK, Ong LK, Levi EM, Charnley JL, Smith DW et al (2014). mTORC1 inhibition in the nucleus accumbens 'protects' against the expression of drug seeking and 'relapse' and is associated with reductions in GluA1 AMPAR and CAMKIIalpha levels. Neuropsychopharmacology 39: 1694–1702.
James MH, Quinn RK, Ong LK, Levi EM, Smith DW, Dickson PW et al (2016). Rapamycin reduces motivated responding for cocaine and alters GluA1 expression in the ventral but not dorsal striatum. Eur J Pharmacol 784: 147–154.
Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R et al (2002). Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci USA 99: 2320–2325.
Laplante M, Sabatini DM (2012). mTOR signaling in growth control and disease. Cell 149: 274–293.
Liu QS, Pu L, Poo MM (2005). Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature 437: 1027–1031.
Liu X, Chen Y, Tong J, Reynolds AM, Proudfoot SC, Qi J et al (2016). Epac signaling is required for cocaine-induced change in AMPA receptor subunit composition in the ventral tegmental area. J Neurosci 36: 4802–4815.
Luikart BW, Schnell E, Washburn EK, Bensen AL, Tovar KR, Westbrook GL (2011). Pten knockdown in vivo increases excitatory drive onto dentate granule cells. J Neurosci 31: 4345–4354.
Luo YX, Han H, Shao J, Gao Y, Yin X, Zhu WL et al (2016). mTOR signalling in the nucleus accumbens shell is critical for augmented effect of TFF3 on behavioural response to cocaine. Sci Rep 6: 27895.
Mameli M, Balland B, Lujan R, Luscher C (2007). Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317: 530–533.
Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R et al (2009). Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12: 1036–1041.
Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M et al (2011). Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron 72: 977–990.
Neasta J, Barak S, Hamida SB, Ron D (2014). mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem 130: 172–184.
Nestler EJ (2013). Cellular basis of memory for addiction. Dialogues Clin Neurosci 15: 431–443.
Nirenberg MJ, Chan J, Pohorille A, Vaughan RA, Uhl GR, Kuhar MJ et al (1997). The dopamine transporter: comparative ultrastructure of dopaminergic axons in limbic and motor compartments of the nucleus accumbens. J Neurosci 17: 6899–6907.
Pan B, Hillard CJ, Liu QS (2008). Endocannabinoid signaling mediates cocaine-induced inhibitory synaptic plasticity in midbrain dopamine neurons. J Neurosci 28: 1385–1397.
Saal D, Dong Y, Bonci A, Malenka RC (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37: 577–582.
Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM (2013). Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front Biosci (Elite Ed) 5: 273–288.
Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM (2014). Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology (Berl) 231: 3109–3118.
Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ (1998). Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci 18: 8003–8015.
Sutton LP, Caron MG (2015). Essential role of D1R in the regulation of mTOR complex1 signaling induced by cocaine. Neuropharmacology 99: 610–619.
Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL (2005). Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci 8: 1727–1734.
Ungless MA, Magill PJ, Bolam JP (2004). Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303: 2040–2042.
Ungless MA, Whistler JL, Malenka RC, Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411: 583–587.
Vialou V, Feng J, Robison AJ, Ku SM, Ferguson D, Scobie KN et al (2012). Serum response factor and cAMP response element binding protein are both required for cocaine induction of DeltaFosB. J Neurosci 32: 7577–7584.
Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF et al (2010). Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30: 12632–12641.
Weston MC, Chen H, Swann JW (2012). Multiple roles for mammalian target of rapamycin signaling in both glutamatergic and GABAergic synaptic transmission. J Neurosci 32: 11441–11452.
Wu J, McCallum SE, Glick SD, Huang Y (2011). Inhibition of the mammalian target of rapamycin pathway by rapamycin blocks cocaine-induced locomotor sensitization. Neuroscience 172: 104–109.
Xiong Q, Oviedo HV, Trotman LC, Zador AM (2012). PTEN regulation of local and long-range connections in mouse auditory cortex. J Neurosci 32: 1643–1652.
Yorgason JT, Espana RA, Jones SR (2011). Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202: 158–164.
Yu F, Zhong P, Liu X, Sun D, Gao HQ, Liu QS (2013). Metabotropic glutamate receptor I (mGluR1) antagonism impairs cocaine-induced conditioned place preference via inhibition of protein synthesis. Neuropsychopharmacology 38: 1308–1321.
Yuan T, Mameli M, O'Connor EC, Dey PN, Verpelli C, Sala C et al (2013). Expression of cocaine-evoked synaptic plasticity by GluN3A-containing NMDA receptors. Neuron 80: 1025–1038.
Zhong P, Liu X, Zhang Z, Hu Y, Liu SJ, Lezama-Ruiz M et al (2014). Cyclin-dependent kinase 5 in the ventral tegmental area regulates depression-related behaviors. J Neurosci 34: 6352–6366.
Zoncu R, Efeyan A, Sabatini DM (2011). mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12: 21–35.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Liu, X., Li, Y., Yu, L. et al. VTA mTOR Signaling Regulates Dopamine Dynamics, Cocaine-Induced Synaptic Alterations, and Reward. Neuropsychopharmacol. 43, 1066–1077 (2018). https://doi.org/10.1038/npp.2017.247
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.247
This article is cited by
-
Paeonol Mitigates Neurodegeneration: Molecular Insights into Its Anti-inflammatory, Antioxidant, and Synaptoprotective Mechanisms
Molecular Neurobiology (2026)
-
Neuroinflammation and pathways that contribute to tourette syndrome
Italian Journal of Pediatrics (2025)
-
Epac2-mediated synaptic insertion of Ca2+-permeable AMPARs in the nucleus accumbens contributes to incubation of cocaine craving
Neuropsychopharmacology (2025)
-
Psilocybin analog 4-OH-DiPT enhances fear extinction and GABAergic inhibition of principal neurons in the basolateral amygdala
Neuropsychopharmacology (2024)
-
Synaptic and mitochondrial mechanisms behind alcohol-induced imbalance of excitatory/inhibitory synaptic activity and associated cognitive and behavioral abnormalities
Translational Psychiatry (2024)