Abstract
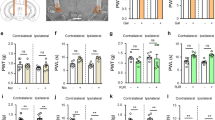
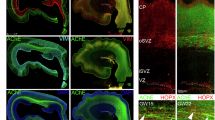
Humans with 15q13.3 microdeletion syndrome (15q13.3DS) are typically hemizygous for CHRNA7, the gene coding for the α7 nicotinic acetylcholine receptor (nAChR), and manifest a variable neuropsychiatric phenotype that frequently includes persistent aggression. In mice, nAChR activation by nicotine is anti-aggressive, or ‘serenic,’ an effect which requires α7 nAChRs and is recapitulated by GTS-21, an α7 nAChR partial agonist. Pharmacotherapies potentiating α7 nAChR signaling have also been shown to reduce aggression in human 15q13.3DS. These findings identify the α7 nAChR as an important regulator of aggressive behavior, but the underlying neurobiological substrates remain to be determined. We therefore investigated the brain regions and potential neural circuits in which α7 nAChRs regulate aggressive behavior in male mice. As in 15q13.3DS, mice heterozygous for Chrna7 were significantly more aggressive compared to wild-type controls in the resident-intruder test. We subsequently examined the hippocampus, where α7 nAChRs are highly expressed, particularly in GABAergic interneurons. Resident–intruder interactions strongly activated granule cells in the dentate gyrus (DG). In contrast, GTS-21, which reduces aggression in mice, reduced DG granule cell activity during resident–intruder interactions. Short hairpin RNA knockdown of Chrna7 in the DG enhanced baseline aggression and eliminated the serenic effects of both nicotine and GTS-21 on attack latency. These data further implicate α7 nAChRs in regulation of aggression, and demonstrate that hippocampal α7 nAChR signaling is necessary and sufficient to limit aggression. These findings suggest that nAChR-mediated regulation of hippocampal excitatory–inhibitory balance could be a promising therapeutic intervention for aggression arising in certain forms of neuropsychiatric disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Baddick CG, Marks MJ (2011). An autoradiographic survey of mouse brain nicotinic acetylcholine receptors defined by null mutants. Biochem Pharmacol 82: 828–841.
Carmel H, Sheitman BB (2007). Adjunctive transdermal nicotine reduced behavioral agitation in severe dementia. Am J Geriatr Psychiatry 15: 449.
Cubells JF, Deoreo EH, Harvey PD, Garlow SJ, Garber K, Adam MP et al (2011). Pharmaco-genetically guided treatment of recurrent rage outbursts in an adult male with 15q13.3 deletion syndrome. Am J Med Genet A 155A: 805–810.
Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB et al (2014). A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry 76: 128–137.
Frazier CJ, Strowbridge BW, Papke RL (2003). Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol 89: 3018–3028.
Freedman R (2014). Alpha7-nicotinic acetylcholine receptor agonists for cognitive enhancement in schizophrenia. Annu Rev Med 65: 245–261.
Freedman R, Wetmore C, Stromberg I, Leonard S, Olson L (1993). Alpha-bungarotoxin binding to hippocampal interneurons: immunocytochemical characterization and effects on growth factor expression. J Neurosci 13: 1965–1975.
Gao R, Penzes P (2015). Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr Mol Med 15: 146–167.
Gillentine MA, White JJ, Grochowski CM, Lupski JR, Schaaf CP, Calarge CA (2017). CHRNA7 deletions are enriched in risperidone-treated children and adolescents. J Child Adolesc Psychopharmacol.
Hoppman-Chaney N, Wain K, Seger PR, Superneau DW, Hodge JC (2013). Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. Clin Genet 83: 345–351.
Hoptman MJ, Antonius D, Mauro CJ, Parker EM, Javitt DC (2014). Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. Am J Psychiatry 171: 939–948.
Hoptman MJ, D'Angelo D, Catalano D, Mauro CJ, Shehzad ZE, Kelly AM et al (2010). Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophr Bull 36: 1020–1028.
Ji D, Dani JA (2000). Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol 83: 2682–2690.
Kohl C, Riccio O, Grosse J, Zanoletti O, Fournier C, Schmidt MV et al (2013). Hippocampal neuroligin-2 overexpression leads to reduced aggression and inhibited novelty reactivity in rats. PLoS ONE 8: e56871.
Koolhaas JM, Coppens CM, de Boer SF, Buwalda B, Meerlo P, Timmermans PJ (2013). The resident-intruder paradigm: a standardized test for aggression, violence and social stress. J Vis Exp 77: e4367.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A et al (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176.
Lewis AS, Mineur YS, Smith PH, Cahuzac EL, Picciotto MR (2015). Modulation of aggressive behavior in mice by nicotinic receptor subtypes. Biochem Pharmacol 97: 488–497.
Lindzey G, Manosevitz M, Winston H (1966). Social dominance in the mouse. Psychon Sci 5: 451–452.
Marks MJ, Burch JB, Collins AC (1983). Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther 226: 291–302.
Masurel-Paulet A, Andrieux J, Callier P, Cuisset JM, Le Caignec C, Holder M et al (2010). Delineation of 15q13.3 microdeletions. Clin Genet 78: 149–161.
Mikhail FM, Lose EJ, Robin NH, Descartes MD, Rutledge KD, Rutledge SL et al (2011). Clinically relevant single gene or intragenic deletions encompassing critical neurodevelopmental genes in patients with developmental delay, mental retardation, and/or autism spectrum disorders. Am J Med Genet A 155A: 2386–2396.
Mineur YS, Fote GM, Blakeman S, Cahuzac EL, Newbold SA, Picciotto MR (2016). Multiple nicotinic acetylcholine receptor subtypes in the mouse amygdala regulate affective behaviors and response to social stress. Neuropsychopharmacology 41: 1579–1587.
Nelson SB, Valakh V (2015). Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87: 684–698.
Nilsson SR, Celada P, Fejgin K, Thelin J, Nielsen J, Santana N et al (2016). A mouse model of the 15q13.3 microdeletion syndrome shows prefrontal neurophysiological dysfunctions and attentional impairment. Psychopharmacology 233: 2151–2163.
Olincy A, Blakeley-Smith A, Johnson L, Kem WR, Freedman R (2016). Brief report: initial trial of alpha7-nicotinic receptor stimulation in two adult patients with autism spectrum disorder. J Autism Dev Disord 46: 3812–3817.
Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D et al (2006). Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry 63: 630–638.
Orr-Urtreger A, Goldner FM, Saeki M, Lorenzo I, Goldberg L, De Biasi M et al (1997). Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J Neurosci 17: 9165–9171.
Picciotto MR, Lewis AS, van Schalkwyk GI, Mineur YS (2015). Mood and anxiety regulation by nicotinic acetylcholine receptors: a potential pathway to modulate aggression and related behavioral states. Neuropharmacology 96 (Pt B): 235–243.
Rissman RA, Mobley WC (2011). Implications for treatment: GABAA receptors in aging, down syndrome and Alzheimer's disease. J Neurochem 117: 613–622.
Rosin RA, Levine MD, Peskind E (2001). Transdermal nicotine for agitation in dementia. Am J Geriatr Psychiatry 9: 443–444.
Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E et al (1994). Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci 6: 1596–1604.
Sala M, Caverzasi E, Lazzaretti M, Morandotti N, De Vidovich G, Marraffini E et al (2011). Dorsolateral prefrontal cortex and hippocampus sustain impulsivity and aggressiveness in borderline personality disorder. J Affect Disord 131: 417–421.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T et al (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682.
Schneider R, Hoffmann HJ, Schicknick H, Moutier R (1992). Genetic analysis of isolation-induced aggression. I. Comparison between closely related inbred mouse strains. Behav Neural Biol 57: 198–204.
Sharp AJ, Mefford HC, Li K, Baker C, Skinner C, Stevenson RE et al (2008). A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet 40: 322–328.
Shinawi M, Schaaf CP, Bhatt SS, Xia Z, Patel A, Cheung SW et al (2009). A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat Genet 41: 1269–1271.
Siegel A, Flynn JP (1968). Differential effects of electrical stimulation and lesions of the hippocampus and adjacent regions upon attack behavior in cats. Brain Res 7: 252–267.
Snyder JS, Radik R, Wojtowicz JM, Cameron HA (2009). Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus 19: 360–370.
Son JH, Winzer-Serhan UH (2008). Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol 511: 286–299.
Spielmann M, Reichelt G, Hertzberg C, Trimborn M, Mundlos S, Horn D et al (2011). Homozygous deletion of chromosome 15q13.3 including CHRNA7 causes severe mental retardation, seizures, muscular hypotonia, and the loss of KLF13 and TRPM1 potentially cause macrocytosis and congenital retinal dysfunction in siblings. Eur J Med Genet 54: e441–e445.
Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF et al (2010). Functional magnetic resonance imaging of effects of a nicotinic agonist in schizophrenia. Neuropsychopharmacology 35: 938–942.
Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R (2004). Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry 161: 315–321.
Unger EK, Burke KJ Jr, Yang CF, Bender KJ, Fuller PM, Shah NM (2015). Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep 10: 453–462.
van der Kooij MA, Fantin M, Kraev I, Korshunova I, Grosse J, Zanoletti O et al (2014). Impaired hippocampal neuroligin-2 function by chronic stress or synthetic peptide treatment is linked to social deficits and increased aggression. Neuropsychopharmacology 39: 1148–1158.
Van Schalkwyk GI, Lewis AS, Qayyum Z, Koslosky K, Picciotto MR, Volkmar FR (2015). Reduction of aggressive episodes after repeated transdermal nicotine administration in a hospitalized adolescent with autism spectrum disorder. J Autism Dev Disord 45: 3061–3066.
Vermeiren Y, Van Dam D, Aerts T, Engelborghs S, De Deyn PP (2014). Monoaminergic neurotransmitter alterations in postmortem brain regions of depressed and aggressive patients with Alzheimer's disease. Neurobiol Aging 35: 2691–2700.
Yin J, Chen W, Yang H, Xue M, Schaaf CP (2017). Chrna7 deficient mice manifest no consistent neuropsychiatric and behavioral phenotypes. Sci Rep 7: 39941.
Zimmerman DW, Zumbo BD (1993). Relative power of the Wilcoxon test, the Friedman test, and repeated-measures ANOVA on ranks. J Exp Educ 62: 75–86.
Acknowledgements
We thank Samantha Sheppard and Nadia Jordan-Spasov for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Lewis, A., Pittenger, S., Mineur, Y. et al. Bidirectional Regulation of Aggression in Mice by Hippocampal Alpha-7 Nicotinic Acetylcholine Receptors. Neuropsychopharmacol. 43, 1267–1275 (2018). https://doi.org/10.1038/npp.2017.276
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.276
This article is cited by
-
Dysfunction of AMPA receptor GluA3 is associated with aggressive behavior in human
Molecular Psychiatry (2022)
-
Regulation of impulsive and aggressive behaviours by a novel lncRNA
Molecular Psychiatry (2021)
-
Posterior amygdala regulates sexual and aggressive behaviors in male mice
Nature Neuroscience (2020)
-
The α7 nicotinic acetylcholine receptors regulate hippocampal adult-neurogenesis in a sexually dimorphic fashion
Brain Structure and Function (2019)
-
An Exploratory Trial of Transdermal Nicotine for Aggression and Irritability in Adults with Autism Spectrum Disorder
Journal of Autism and Developmental Disorders (2018)