Abstract
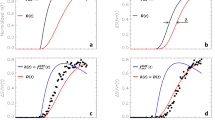
Methamphetamine (METH) is a drug with a high addictive potential that is widely abused across the world. Although it is known that METH dysregulates both dopamine transmission and dopamine reuptake, the specific mechanism of action remains obscure. One promising target of METH is the sigma receptor, a chaperone protein located on the membrane of the endoplasmic reticulum. Using fast-scan cyclic voltammetry, we show that METH-enhancement of evoked dopamine release and basal efflux is dependent on sigma receptor activation. METH-induced activation of sigma receptors results in oxidation of a cysteine residue on VMAT2, which decreases transporter function. Unilateral injections of the sigma receptor antagonist BD-1063 prior to METH administration increased dopamine-related ipsilateral circling behavior, indicating the involvement of sigma receptors. These findings suggest that interactions between METH and the sigma receptor lead to oxidative species (most likely superoxide) that in turn oxidize VMAT2. Altogether, these findings show that the sigma receptor has a key role in METH dysregulation of dopamine release and dopamine-related behaviors.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ali SF, Kordsmeier KJ, Gough B (1995). Drug-induced circling preference in rats. Correlation with monoamine levels. Mol Neurobiol 11: 145–154.
Barbosa DJ, Capela JP, Feio-Azevedo R, Teixeira-Gomes A, Bastos Mde L, Carvalho F (2015). Mitochondria: key players in the neurotoxic effects of amphetamines. Arch Toxicol 89: 1695–1725.
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009). Regulation and function of selenoproteins in human disease. Biochem J 422: 11–22.
Casida JE, Ford B, Jinsmaa Y, Sullivan P, Cooney A, Goldstein DS (2014). Benomyl, aldehyde dehydrogenase, DOPAL, and the catecholaldehyde hypothesis for the pathogenesis of Parkinson's disease. Chem Res Toxicol 27: 1359–1361.
Chu PW, Seferian KS, Birdsall E, Truong JG, Riordan JA, Metcalf CS et al (2008). Differential regional effects of methamphetamine on dopamine transport. Eur J Pharmacol 590: 105–110.
Eshleman AJ, Henningsen RA, Neve KA, Janowsky A (1994). Release of dopamine via the human transporter. Molecular Pharmacology 45: 312–316.
Fernandes VM, Romano-Silva MA, Gomes DA, Prado MA, Santos TM, Gomez MV (2004). Dopamine release evoked by beta scorpion toxin, tityus gamma, in prefrontal cortical slices is mediated by intracellular calcium stores. Cell Mol Neurobiol 24: 757–767.
Fleckenstein AE, Volz TJ, Hanson GR (2009). Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology 56 (Suppl 1): 133–138.
Floor E, Leventhal PS, Schaeffer SF (1990). Partial purification and characterization of the vacuolar H(+)-ATPase of mammalian synaptic vesicles. J Neurochem 55: 1663–1670.
Friend DM, Fricks-Gleason AN, Keefe KA (2014). Is there a role for nitric oxide in methamphetamine-induced dopamine terminal degeneration? Neurotox Res 25: 153–160.
Freyberg Z, Sonders MS, Aguilar JI, Hiranita T, Karam CS, Flores J et al (2016). Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat Commun 7: 10652.
Fukunaga K, Shinoda Y, Tagashira H (2015). The role of SIGMAR1 gene mutation and mitochondrial dysfunction in amyotrophic lateral sclerosis. J Pharmacol Sci 127: 36–41.
Gantz SC, Bunzow JR, Williams JT (2013). Spontaneous inhibitory synaptic currents mediated by a g protein-coupled receptor. Neuron 78: 807–812.
Han DD, Gu HH (2006). Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol 6: 6.
Hartung H, Threlfell S, Cragg SJ (2011). Nitric oxide donors enhance the frequency dependence of dopamine release in nucleus accumbens. Neuropsychopharmacology 36: 1811–1822.
Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL (2013). Stimulants as specific inducers of dopamine-independent sigma agonist self-administration in rats. J Pharmacol Exp Ther 347: 20–29.
Huang MC, Lin SK, Chen CH, Pan CH, Lee CH, Liu HC (2013). Oxidative stress status in recently abstinent methamphetamine abusers. Psychiatry Clin Neurosci 67: 92–100.
Ikeda H, Kotani A, Koshikawa N, Cools AR (2007). A vehicle injection into the right core of the nucleus accumbens both reverses the region-specificity and alters the type of contralateral turning elicited by unilateral stimulation of dopamine D2/D3 and D1 receptors in the left core of the nucleus accumbens. Eur J Pharmacol 577: 64–70.
Imam SZ, el Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W Jr. et al (2001a). Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann N Y Acad Sci 939: 366–380.
Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W Jr. et al (2001b). Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem 76: 745–749.
Ingram SL, Prasad BM, Amara SG (2002). Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci 5: 971–978.
Itzhak Y (1993). Repeated methamphetamine-treatment alters brain sigma receptors. Eur J Pharmacol 230: 243–244.
Jalewa J, Sharma MK, Gengler S, Holscher C (2017). A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson's disease. Neuropharmacology 117: 238–248.
Jang EY, Ryu YH, Lee BH, Chang SC, Yeo MJ, Kim SH et al (2015). Involvement of reactive oxygen species in cocaine-taking behaviors in rats. Addict Biol 20: 663–675.
Jang EY, Yang CH, Hedges DM, Kim SP, Lee JY, Ekins TG et al (2017). The role of reactive oxygen species in methamphetamine self-administration and dopamine release in the nucleus accumbens. Addict Biol. 22: 1304–1315.
Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J et al (2006). Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology 185: 327–338.
John CE, Jones SR (2007). Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology 52: 1596–1605.
Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG (1998a). Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 95: 4029–4034.
Jones SR, Gainetdinov RR, Wightman RM, Caron MG (1998b). Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci 18: 1979–1986.
Kataoka Y, Koizumi S, Niwa M, Shibaguchi H, Shigematsu K, Kudo Y et al (1994). Endothelin-3 stimulates inositol 1,4,5-trisphosphate production and Ca2+ influx to produce biphasic dopamine release from rat striatal slices. Cell Mol Neurobiol 14: 271–280.
Katz JL, Su TP, Hiranita T, Hayashi T, Tanda G, Kopajtic T et al (2011) A role for sigma receptors in stimulant self administration and addiction Pharmaceuticals Basel 4: 880–914.
Kaushal N, Elliott M, Robson MJ, Iyer AK, Rojanasakul Y, Coop A et al (2012). AC927, a sigma receptor ligand, blocks methamphetamine-induced release of dopamine and generation of reactive oxygen species in NG108-15 cells. Mol Pharmacol 81: 299–308.
Kita T, Takahashi M, Kubo K, Wagner GC, Nakashima T (1999). Hydroxyl radical formation following methamphetamine administration to rats. Pharmacol Toxicol 85: 133–137.
Knutson B, Bjork JM, Fong GW, Hommer D, Mattay VS, Weinberger DR (2004). Amphetamine modulates human incentive processing. Neuron 43: 261–269.
LaVoie MJ, Hastings TG (1999). Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci 19: 1484–1491.
Lohr KM, Stout KA, Dunn AR, Wang M, Salahpour A, Guillot TS et al (2015). Increased vesicular monoamine transporter 2 (VMAT2; Slc18a2) protects against methamphetamine toxicity. ACS Chem Neurosci 6: 790–799.
Matsumoto RR, Nguyen L, Kaushal N, Robson MJ (2014). Sigma (sigma) receptors as potential therapeutic targets to mitigate psychostimulant effects. Adv Pharmacol 69: 323–386.
Maurice T, Su TP (2009). The pharmacology of sigma-1 receptors. Pharmacol Ther 124: 195–206.
McFadden LM, Vieira-Brock PL, Hanson GR, Fleckenstein AE (2015). Prior methamphetamine self-administration attenuates the dopaminergic deficits caused by a subsequent methamphetamine exposure. Neuropharmacology 93: 146–154.
Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR (2005). Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology 49: 638–645.
Nguyen L, Kaushal N, Robson MJ, Matsumoto RR (2014). Sigma receptors as potential therapeutic targets for neuroprotection. Eur J Pharmacol 743: 42–47.
Robinson TE, Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291.
Rossi R, Giustarini D, Dalle-Donne I, Milzani A (2006). Protein S-glutathionylation and platelet anti-aggregating activity of disulfiram. Biochem Pharmacol 72: 608–615.
Rothman RB, Baumann MH (2003). Monoamine transporters and psychostimulant drugs. Eur J Pharmacol 479: 23–40.
Ruscher K, Wieloch T (2015). The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration. J Pharmacol Sci 127: 30–35.
Saigusa T, Koshikawa N, Kitamura M, Kobayashi M (1993). Reevaluation of the two-component hypothesis for turning behaviour by manipulating activities in the striatum and the nucleus accumbens of intact rats. Eur J Pharmacol 237: 161–168.
Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D (2001). Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci 21: 5916–5924.
Shiba T, Yamato M, Kudo W, Watanabe T, Utsumi H, Yamada K (2011). In vivo imaging of mitochondrial function in methamphetamine-treated rats. Neuroimage 57: 866–872.
Siciliano CA, Calipari ES, Ferris MJ, Jones SR (2014). Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci 34: 5575–5582.
Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C (1998). Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem 71: 1289–1297.
Solhi H, Malekirad A, Kazemifar AM, Sharifi F (2014). Oxidative stress and lipid peroxidation in prolonged users of methamphetamine. Drug Metab Lett 7: 79–82.
Steffensen SC, Taylor SR, Horton ML, Barber EN, Lyle LT, Stobbs SH et al (2008). Cocaine disinhibits dopamine neurons in the ventral tegmental area via use-dependent blockade of GABA neuron voltage-sensitive sodium channels. Eur J Neurosci 28: 2028–2040.
Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE (2010). The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci 31: 557–566.
Sulzer D (2011). How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69: 628–649.
Sulzer D, Sonders MS, Poulsen NW, Galli A (2005). Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75: 406–433.
Uys JD, Mulholland PJ, Townsend DM (2014). Glutathione and redox signaling in substance abuse. Biomed Pharmacother 68: 799–807.
Walker J, Winhusen T, Storkson JM, Lewis D, Pariza MW, Somoza E et al (2014). Total antioxidant capacity is significantly lower in cocaine-dependent and methamphetamine-dependent patients relative to normal controls: results from a preliminary study. Hum Psychopharmacol 29: 537–543.
Womersley JS, Uys JD (2016). S-glutathionylation and redox protein signaling in drug addiction. Prog Mol Biol Transl Sci 137: 87–121.
Wyvell CL, Berridge KC (2000). Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward "wanting" without enhanced "liking" or response reinforcement. J Neurosci 20: 8122–8130.
Yorgason JT, Espana RA, Jones SR (2011). Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202: 158–164.
Yorgason JT, Zeppenfeld DM, Williams JT (2017). Cholinergic interneurons underlie spontaneous dopamine release in nucleus accumbens. J Neurosci 37: 2086–2096.
Yu S, Zhu L, Shen Q, Bai X, Di X (2015). Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol 2015: 103969.
Acknowledgements
We would like to thank the personnel who contributed to this project, namely Dr. Jacqueline Womersley, Dr. Bryan Blummel, Andrew Perez, Christopher Schow, Gilbert Marchant, Spencer McCarthy, and Mark Woodbury. We would like to dedicate this work in memory of Samuel I. Shin.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Hedges, D., Obray, J., Yorgason, J. et al. Methamphetamine Induces Dopamine Release in the Nucleus Accumbens Through a Sigma Receptor-Mediated Pathway. Neuropsychopharmacol. 43, 1405–1414 (2018). https://doi.org/10.1038/npp.2017.291
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.291
This article is cited by
-
Methamphetamine-Driven Neuroinflammation and Parkinson’s Disease Pathology: Mechanistic Insight into Nrf2 and NFĸB Signaling
Molecular Neurobiology (2026)
-
Prefrontal circHomer1 regulates synaptic and behavioral adaptations induced by cocaine
Molecular Psychiatry (2025)
-
Biphasic Model of Addiction: Neurobehavioral Adaptations
Current Behavioral Neuroscience Reports (2025)
-
CNTN1 in the Nucleus Accumbens is Involved in Methamphetamine-Induced Conditioned Place Preference in Mice
Neurotoxicity Research (2023)
-
A novel microRNA, novel-m009C, regulates methamphetamine rewarding effects
Molecular Psychiatry (2022)