Abstract
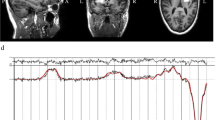
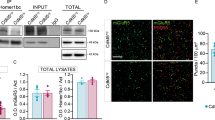
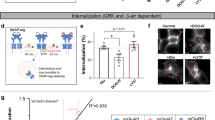
Drugs targeting metabotropic glutamate receptor 5 (mGluR5) have therapeutic potential in autism spectrum disorders (ASD), including tuberous sclerosis complex (TSC). The question whether inhibition or potentiation of mGluR5 could be beneficial depends, among other factors, on the specific indication. To facilitate the development of mGluR5 treatment strategies, we tested the therapeutic utility of mGluR5 negative and positive allosteric modulators (an mGluR5 NAM and PAM) for TSC, using a mutant mouse model with neuronal loss of Tsc2 that demonstrates disease-related phenotypes, including behavioral symptoms of ASD and epilepsy. This model uniquely enables the in vivo characterization and rescue of the electrographic seizures associated with TSC. We demonstrate that inhibition of mGluR5 corrects hyperactivity, seizures, and elevated de novo synaptic protein synthesis. Conversely, positive allosteric modulation of mGluR5 results in the exacerbation of hyperactivity and epileptic phenotypes. The data suggest a meaningful therapeutic potential for mGluR5 NAMs in TSC, which warrants clinical exploration and the continued development of mGluR5 therapies.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S et al (2006). Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med 47: 698–705.
Auerbach BD, Osterweil EK, Bear MF (2011). Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature 480: 63–68.
Davis PE, Peters JM, Krueger DA, Sahin M (2015). Tuberous sclerosis: a new frontier in targeted treatment of autism. Neurotherapeutics 12: 572–583.
de Vries PJ, Whittemore VH, Leclezio L, Byars AW, Dunn D, Ess KC et al (2015). Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol 52: 25–35.
Dennis PB, Fumagalli S, Thomas G (1999). Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Curr Opin Genet Dev 9: 49–54.
Dhamne SC, Silverman JL, Super CE, Lammers SHT, Hameed MQ, Modi ME et al (2017). Replicable in vivo physiological and behavioral phenotypes of the Shank3B null mutant mouse model of autism. Mol Autism 8: 26.
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ et al (2008). Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med 14: 843–848.
Gilmour G, Broad LM, Wafford KA, Britton T, Colvin EM, Fivush A et al (2013). In vitro characterisation of the novel positive allosteric modulators of the mGlu(5) receptor, LSN2463359 and LSN2814617, and their effects on sleep architecture and operant responding in the rat. Neuropharmacology 64: 224–239.
Hintermann S, Vranesic I, Allgeier H, Brulisauer A, Hoyer D, Lemaire M et al (2007). ABP688, a novel selective and high affinity ligand for the labeling of mGlu5 receptors: identification, in vitro pharmacology, pharmacokinetic and biodistribution studies. Bioorg Med Chem 15: 903–914.
Hoeffer CA, Klann E (2010). mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci 33: 67–75.
Holmes A, Hollon TR, Gleason TC, Liu Z, Dreiling J, Sibley DR et al (2001). Behavioral characterization of dopamine D5 receptor null mutant mice. Behav Neurosci 115: 1129–1144.
Kinney GG, O'Brien JA, Lemaire W, Burno M, Bickel DJ, Clements MK et al (2005). A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther 313: 199–206.
Laplante M, Sabatini DM (2012). mTOR signaling in growth control and disease. Cell 149: 274–293.
Lindemann L, Jaeschke G, Michalon A, Vieira E, Honer M, Spooren W et al (2011). CTEP: a novel, potent, long-acting, and orally bioavailable metabotropic glutamate receptor 5 inhibitor. J Pharmacol Exp Ther 339: 474–486.
Lipton JO, Sahin M (2014). The neurology of mTOR. Neuron 84: 275–291.
Mares P, Mikulecka A, Ticha K, Lojkova-Janeckova D, Kubova H (2010). Metabotropic glutamate receptors as a target for anticonvulsant and anxiolytic action in immature rats. Epilepsia 51 (Suppl 3): 24–26.
Michalon A, Bruns A, Risterucci C, Honer M, Ballard TM, Ozmen L et al (2014). Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol Psychiatry 75: 189–197.
Michalon A, Sidorov M, Ballard TM, Ozmen L, Spooren W, Wettstein JG et al (2012). Chronic pharmacological mGlu5 inhibition corrects fragile X in adult mice. Neuron 74: 49–56.
Nie D, Chen Z, Ebrahimi-Fakhari D, Di Nardo A, Julich K, Robson VK et al (2015). The stress-induced Atf3-gelsolin cascade underlies dendritic spine deficits in neuronal models of tuberous sclerosis complex. J Neurosci 35: 10762–10772.
Parmentier-Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O'Brien JA et al (2014). Mechanism based neurotoxicity of mGlu5 positive allosteric modulators—development challenges for a promising novel antipsychotic target. Neuropharmacology 82: 161–173.
Pollizzi K, Malinowska-Kolodziej I, Doughty C, Betz C, Ma J, Goto J et al (2009). A hypomorphic allele of Tsc2 highlights the role of TSC1/TSC2 in signaling to AKT and models mild human TSC2 alleles. Hum Mol Genet 18: 2378–2387.
Potter WB, Basu T, O'Riordan KJ, Kirchner A, Rutecki P, Burger C et al (2013). Reduced juvenile long-term depression in tuberous sclerosis complex is mitigated in adults by compensatory recruitment of mGluR5 and Erk signaling. PLoS Biol 11: e1001627.
Schmidt EK, Clavarino G, Ceppi M, Pierre P (2009). SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 6: 275–277.
Seese RR, Maske AR, Lynch G, Gall CM (2014). Long-term memory deficits are associated with elevated synaptic ERK1/2 activation and reversed by mGluR5 antagonism in an animal model of autism. Neuropsychopharmacology 39: 1664–1673.
Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS et al (2012). Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med 4: 131ra151.
Spooren W, Gasparini F (2004). mGlu5 receptor antagonists: a novel class of anxiolytics? Drug News Perspect 17: 251–257.
Tian D, Stoppel LJ, Heynen AJ, Lindemann L, Jaeschke G, Mills AA et al (2015). Contribution of mGluR5 to pathophysiology in a mouse model of human chromosome 16p11.2 microdeletion. Nat Neurosci 18: 182–184.
Treyer V, Streffer J, Ametamey SM, Bettio A, Blauenstein P, Schmidt M et al (2008). Radiation dosimetry and biodistribution of 11C-ABP688 measured in healthy volunteers. Eur J Nucl Med Mol Imaging 35: 766–770.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM et al (2012). Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488: 647–651.
Yuan E, Tsai PT, Greene-Colozzi E, Sahin M, Kwiatkowski DJ, Malinowska IA (2012). Graded loss of tuberin in an allelic series of brain models of TSC correlates with survival, and biochemical, histological and behavioral features. Hum Mol Genet 21: 4286–4300.
Acknowledgements
We are indebted to members of the Sahin lab for critical reading of the manuscript.
This study was supported by the BCH Neurodevelopmental Behavioral Core of the Intellectual and Developmental Disabilities Research Center (NIH U54 HD090255), the BCH Translational Neuroscience Center, and the Experimental Neurophysiology Core, Nancy Lurie Marks Family Foundation, and the Boston Children’s Hospital Translational Research Program (to M.S. and A.R.).
Author contributions
Conceived and designed the experiments: A.R., L.L., and M.S.; performed the experiments: E.K., S.M.S., S.C.D., J.O.L., C.E.S., S.H.T.L., and M.H.; analyzed the data: S.C.D., J.O.L., M.E.M., A.R., M.H., and M.S.; contributed reagents/materials/analysis tools: D.J.K., L.L., G.J., J.L.S., and J.R.D.; drafted the article: E.K., S.M.S., S.C.D., L.L., M.E.M., and M.S.; revised the article: E.K., S.M.S., S.C.D., J.O.L., L.L., C.E.S., S.H.T., M.E.M., J.L.S., J.R.D, D.J.K., A.R., and M.S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Kelly, E., Schaeffer, S., Dhamne, S. et al. mGluR5 Modulation of Behavioral and Epileptic Phenotypes in a Mouse Model of Tuberous Sclerosis Complex. Neuropsychopharmacol. 43, 1457–1465 (2018). https://doi.org/10.1038/npp.2017.295
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.295
This article is cited by
-
mGluR5 PAMs rescue cortical and behavioural defects in a mouse model of CDKL5 deficiency disorder
Neuropsychopharmacology (2023)
-
The research landscape of tuberous sclerosis complex–associated neuropsychiatric disorders (TAND)—a comprehensive scoping review
Journal of Neurodevelopmental Disorders (2022)
-
Abnormal electrophysiological phenotypes and sleep deficits in a mouse model of Angelman Syndrome
Molecular Autism (2021)
-
Regulation of lifespan by neural excitation and REST
Nature (2019)
-
A framework for the investigation of rare genetic disorders in neuropsychiatry
Nature Medicine (2019)