Abstract
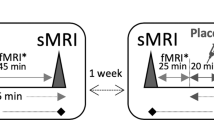
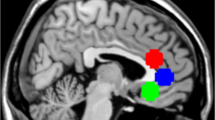
Animal models of depression repeatedly showed stress-induced nucleus accumbens (NAc) hypertrophy. Recently, ketamine was found to normalize this stress-induced NAc structural growth. Here, we investigated NAc structural abnormalities in major depressive disorder (MDD) in two cohorts. Cohort A included a cross-sectional sample of 34 MDD and 26 healthy control (HC) subjects, with high-resolution magnetic resonance imaging (MRI) to estimate NAc volumes. Proton MR spectroscopy (1H MRS) was used to divide MDD subjects into two subgroups: glutamate-based depression (GBD) and non-GBD. A separate longitudinal sample (cohort B) included 16 MDD patients who underwent MRI at baseline then 24 h following intravenous infusion of ketamine (0.5 mg/kg). In cohort A, we found larger left NAc volume in MDD compared to controls (Cohen’s d=1.05), but no significant enlargement in the right NAc (d=0.44). Follow-up analyses revealed significant subgrouping effects on the left (d⩾1.48) and right NAc (d⩾0.95) with larger bilateral NAc in non-GBD compared to GBD and HC. NAc volumes were not different between GBD and HC. In cohort B, ketamine treatment reduced left NAc, but increased left hippocampal, volumes in patients achieving remission. The cross-sectional data provided the first evidence of enlarged NAc in patients with MDD. These NAc abnormalities were limited to patients with non-GBD. The pilot longitudinal data revealed a pattern of normalization of left NAc and hippocampal volumes particularly in patients who achieved remission following ketamine treatment, an intriguing preliminary finding that awaits replication.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Abdallah CG, Adams TG, Kelmendi B, Esterlis I, Sanacora G, Krystal JH (2016a). Ketamine's mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety 33: 689–697.
Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C et al (2016b). Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacology (doi:10.1038/npp.2016.186).
Abdallah CG, Coplan JD, Jackowski A, Sato JR, Mao X, Shungu DC et al (2013). A pilot study of hippocampal volume and N-acetylaspartate (NAA) as response biomarkers in riluzole-treated patients with GAD. Eur Neuropsychopharmacol 23: 276–284.
Abdallah CG, Jackowski A, Sato JR, Mao X, Kang G, Cheema R et al (2015a). Prefrontal cortical GABA abnormalities are associated with reduced hippocampal volume in major depressive disorder. Eur Neuropsychopharmacol 25: 1082–1090.
Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL et al (2014). Glutamate Metabolism in Major Depressive Disorder. Am J Psychiatry 171: 1320–1327.
Abdallah CG, Salas R, Jackowski A, Baldwin P, Sato JR, Mathew SJ (2015b). Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol 29: 591–595.
Abdallah CG, Sanacora G, Duman RS, Krystal JH (2015c). Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 66: 509–523.
Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG (2016). Glutamate dysregulation and glutamatergic therapeutics for PTSD: evidence from human studies. Neurosci Lett (doi:10.1016/j.neulet.2016.11.064).
Belujon P, Grace AA (2014). Restoring mood balance in depression: ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry 76: 927–936.
Burrows-Kerr R. Repetitive negative thought and anhedonia: a systematic review (literature review); Repetitive negative thought and reward sensitivity (empirical paper). University of Exeter: Exeter Devon, UK, 2015..
Campioni MR, Xu M, McGehee DS (2009). Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol 101: 3192–3198.
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW et al (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493: 532–536.
Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF et al (2011). IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31: 314–321.
Christoffel DJ, Golden SA, Heshmati M, Graham A, Birnbaum S, Neve RL et al (2012). Effects of inhibitor of kappaB kinase activity in the nucleus accumbens on emotional behavior. Neuropsychopharmacology 37: 2615–2623.
Coplan JD, Gopinath S, Abdallah CG, Berry BR (2014). A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci 8: 189.
Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016). Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 22: 238–249.
Fischl B (2012). FreeSurfer. Neuroimage 62: 774–781.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C et al (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33: 341–355.
Hawley CJ, Gale TM, Sivakumaran T, Hertfordshire Neuroscience Research group (2002). Defining remission by cut off score on the MADRS: selecting the optimal value. J Affect Disord 72: 177–184.
Kassem MS, Lagopoulos J, Stait-Gardner T, Price WS, Chohan TW, Arnold JC et al (2013). Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol 47: 645–661.
Kempton MJ, Salvador Z, Munafo MR, Geddes JR, Simmons A, Frangou S et al (2011). Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68: 675–690.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ et al (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404.
Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H et al (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69: 754–761.
Li Y, Zhu ZR, Ou BC, Wang YQ, Tan ZB, Deng CM et al (2015). Dopamine D2/D3 but not dopamine D1 receptors are involved in the rapid antidepressant-like effects of ketamine in the forced swim test. Behav Brain Res 279: 100–105.
Liem F, Merillat S, Bezzola L, Hirsiger S, Philipp M, Madhyastha T et al (2015). Reliability and statistical power analysis of cortical and subcortical FreeSurfer metrics in a large sample of healthy elderly. Neuroimage 108: 95–109.
McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN et al (2015). Mechanisms of stress in the brain. Nat Neurosci 18: 1353–1363.
Melo A, Kokras N, Dalla C, Ferreira C, Ventura-Silva AP, Sousa N et al (2015). The positive effect on ketamine as a priming adjuvant in antidepressant treatment. Transl Psychiatry 5: e573.
Muhammad A, Carroll C, Kolb B (2012). Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 216: 103–109.
Munte TF, Heldmann M, Hinrichs H, Marco-Pallares J, Kramer UM, Sturm V et al (2007). Nucleus Accumbens is Involved in Human Action Monitoring: Evidence from Invasive Electrophysiological Recordings. Front Hum Neurosci 1: 11.
Nestler EJ (2015). Role of the Brain's Reward Circuitry in Depression: Transcriptional Mechanisms. Int Rev Neurobiol 124: 151–170.
Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T et al (2015). Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry 172: 124–138.
Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA et al (2009). Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65: 792–800.
Reus GZ, Abelaira HM, dos Santos MA, Carlessi AS, Tomaz DB, Neotti MV et al (2013). Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav Brain Res 256: 451–456.
Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61: 1402–1418.
Russo SJ, Nestler EJ (2013). The brain reward circuitry in mood disorders. Nat Rev Neurosci 14: 609–625.
Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F et al (2010). Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nat Neurosci 13: 920–922.
Wacker J, Dillon DG, Pizzagalli DA (2009). The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage 46: 327–337.
Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B et al (2014). Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci 17: 27–29.
Warren BL, Sial OK, Alcantara LF, Greenwood MA, Brewer JS, Rozofsky JP et al (2014). Altered gene expression and spine density in nucleus accumbens of adolescent and adult male mice exposed to emotional and physical stress. Dev Neurosci 36: 250–260.
Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN et al (2016). The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358: 71–82.
Wook Koo J, Labonte B, Engmann O, Calipari ES, Juarez B, Lorsch Z et al (2016). Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol Psychiatry 80: 469–478.
Acknowledgements
We thank the subjects who participated in this study for their invaluable contribution. This work was supported by the Clinical Neuroscience Division National Center for PTSD, K23 MH-101498, Brain and Behavior Foundation (NARSAD New Investigator Award; CGA), R01 MH07895 (DCS), K23 MH-069656, MO1 RR-00071, R01 MH-081870. The Johnson Family Chair for Research, and by the use of resources and facilities at the Michael E. DeBakey VA Medical Center, Houston, Texas (SJM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Abdallah, C., Jackowski, A., Salas, R. et al. The Nucleus Accumbens and Ketamine Treatment in Major Depressive Disorder. Neuropsychopharmacol 42, 1739–1746 (2017). https://doi.org/10.1038/npp.2017.49
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.49
This article is cited by
-
Effects of psychoplastogens on blood levels of brain-derived neurotrophic factor (BDNF) in humans: a systematic review and meta-analysis
Molecular Psychiatry (2025)
-
Challenges and rewards of in vivo synaptic density imaging, and its application to the study of depression
Neuropsychopharmacology (2025)
-
Single-Nucleus Transcriptomics of the Nucleus Accumbens Reveals Cell-Type-Specific Dysregulation in Adolescent Macaques with Depressive-Like Behaviors
Neuroscience Bulletin (2025)
-
Hippocampal volume changes after (R,S)-ketamine administration in patients with major depressive disorder and healthy volunteers
Scientific Reports (2024)
-
Elevated neuron specific enolase levels in post-traumatic stress disorder
European Journal of Pediatrics (2024)