Abstract
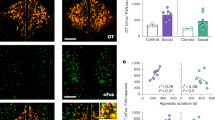
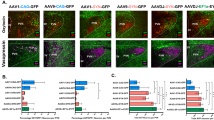
Oxytocin (OXT), synthesized in the hypothalamic paraventricular nucleus (PVN) and then released into different brain areas, may play a crucial role in various behaviors and neuropsychiatric disorders, including depression. Testosterone has been proposed by clinical studies to have the opposite effect of oxytocin in these disorders. We began by studying, in the postmortem hypothalamus of fifteen patients with mood disorders and fifteen matched controls, the expression of OXT in the PVN by means of immunocytochemistry (ICC) and the co-localization of OXT and androgen receptor (AR) by means of double labeling ICC. Subsequently, the regulatory effect of AR on OXT gene expression was studied in vitro. We found a higher expression of PVN OXT in the mood disorder patients than in the control subjects, and observed a clear co-localization of AR in OXT-expressing neurons, both in the cytoplasm and in the nucleus. In addition, a significant decrease in OXT-mRNA levels was observed after pre-incubation of the SK-N-SH cells with testosterone. A further potential androgen-responsive element in the human OXT gene promotor was revealed by electrophoretic mobility shift assays and co-transfections in neuroblastoma cells. Finally, in vitro studies demonstrated that AR mediated the down-regulation of OXT gene expression. These results suggest that the fact that OXT and testosterone appear to have opposite effects in neuropsychiatric disorders might be based upon a direct inhibition of AR on OXT transcription, which may provide a novel target for therapeutic strategies in depression.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bao A, Fischer D, Wu Y, Hol E, Balesar R, Unmehopa U et al (2006). A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry 11: 567–576.
Baxter LC (2016). Appetite changes in depression. Am J Psychiatry 173: 317–318.
Bennett NC, Gardiner RA, Hooper JD, Johnson DW, Gobe GC (2010). Molecular cell biology of androgen receptor signalling. Int J Biochem Cell Biol 42: 813–827.
Bingaman EW, Baeckman LM, Yracheta JM, Handa RJ, Gray TS (1994). Localization of androgen receptor within peptidergic neurons of the rat forebrain. Brain Res Bull 35: 379–382.
Carson DS, Guastella AJ, Taylor ER, McGregor IS (2013). A brief history of oxytocin and its role in modulating psychostimulant effects. J Psychopharmacol 27: 231–247.
Cochran D, Fallon D, Hill M, Frazier JA (2013). The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry 21: 219.
Crespi BJ (2015). Oxytocin, testosterone, and human social cognition. Biol Rev 91: 390–408.
Fernándezguasti A, Kruijver FP, Fodor M, Swaab DF (2000). Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol 425: 422–435.
Filippi S, Luconi M, Granchi S, Vignozzi L, Bettuzzi S, Tozzi P et al (2002). Estrogens, but not androgens, regulate expression and functional activity of oxytocin receptor in rabbit epididymis. Endocrinology 143: 4271–4280.
Fronczek R, Lammers GJ, Balesar R, Unmehopa UA, Swaab DF (2005). The number of hypothalamic hypocretin (orexin) neurons is not affected in Prader-Willi syndrome. J Clin Endocrinol Metab 90: 5466–5470.
Gao SF, Qi XR, Zhao J, Balesar R, Bao AM, Swaab DF (2013). Decreased NOS1 expression in the anterior cingulate cortex in depression. Cerebr Cortex 23: 2956–2964.
Heinrichs M, Domes G (2008). Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res 170: 337–350.
Hiroi R, Lacagnina AF, Hinds LR, Carbone DG, Uht RM, Handa RJ (2013). The androgen metabolite, 5α-androstane-3β, 17β-diol (3β-diol), activates the oxytocin promoter through an estrogen receptor-β pathway. Endocrinology 154: 1802–1812.
Hofmann SG, Fang A, Brager DN (2015). Effect of intranasal oxytocin administration on psychiatric symptoms: a meta-analysis of placebo-controlled studies. Psychiatry Res 228: 708–714.
Humble MB, Uvnäs-Moberg K, Engström I, Bejerot S (2013). Plasma oxytocin changes and anti-obsessive response during serotonin reuptake inhibitor treatment: a placebo controlledstudy. BMC Psychiatry 13: 1.
Karlsson SA, Studer E, Kettunen P, Westberg L (2016). Neural androgen receptors modulate gene expression and social recognition but not social investigation. Front Behav Neurosci 10: 1–13.
Keating C, Dawood T, Barton DA, Lambert GW, Tilbrook AJ (2013). Effects of selective serotonin reuptake inhibitor treatment on plasma oxytocin and cortisol in major depressive disorder. BMC Psychiatry 13: 124.
Knobloch HS, Charlet A, Hoffmann Lena C, Eliava M, Khrulev S, Cetin Ali H et al (2012). Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73: 553–566.
Lawson EA, Holsen LM, Santin M, Meenaghan E, Eddy KT, Becker AE et al (2012). Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. J Clin Endocrinol Metab 97: E1898–E1908.
Legros J-J (2001). Inhibitory effect of oxytocin on corticotrope function in humans: are vasopressin and oxytocin ying-yang neurohormones? Psychoneuroendocrinology 26: 649–655.
Leng G, Ludwig M (2016). Intranasal oxytocin: myths and delusions. Biol Psychiatry 79: 243–250.
McQuaid RJ, McInnis OA, Abizaid A, Anisman H (2014). Making room for oxytocin in understanding depression. Neurosci Biobehav Rev 45: 305–322.
McQuaid RJ, McInnis OA, Matheson K, Anisman H (2016). Oxytocin and social sensitivity: gene polymorphisms in relation to depressive symptoms and suicidal ideation. Front Hum Neurosci 10.
Morris MS, Domino EF, Domino SE (2010). Opioid modulation of oxytocin release. J Clin Pharmacol 50: 1112–1117.
Murakami G, Hunter RG, Fontaine C, Ribeiro A, Pfaff D (2011). Relationships among estrogen receptor, oxytocin and vasopressin gene expression and social interaction in male mice. Eur J Neurosci 34: 469–477.
Need EF, Selth LA, Harris TJ, Birrell SN, Tilley WD, Buchanan G (2012). Research resource: interplay between the genomic and transcriptional networks of androgen receptor and estrogen receptor α in luminal breast cancer cells. Mol Endocrinol 26: 1941–1952.
Ozsoy S, Esel E, Kula M (2009). Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res 169: 249–252.
Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF (1996). Increased number of vasopressin-and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry 53: 137–143.
Quirin M, Kuhl J, Düsing R (2011). Oxytocin buffers cortisol responses to stress in individuals with impaired emotion regulation abilities. Psychoneuroendocrinology 36: 898–904.
Richard SP, Zingg H (1990). The human oxytocin gene promoter is regulated by estrogens. J Biol Chem 265: 6098–6103.
Rizvi SJ, Kennedy SH, Ravindran LN, Giacobbe P, Eisfeld BS, Mancini D et al (2010). The relationship between testosterone and sexual function in depressed and healthy men. J Sexual Med 7: 816–825.
Saatcioglu F Androgen Action: Methods and Protocols. Humana Press: Hatfield, UK, 2011.
Sharma D, Handa RJ, Uht RM (2012). The ERβ ligand 5α-androstane, 3β, 17β-diol (3β-diol) regulates hypothalamic oxytocin (Oxt) gene expression. Endocrinology 153: 2353–2361.
Smets LA, Loesberg C, Janssen M, Metwally EA, Huiskamp R (1989). Active uptake and extravesicular storage of m-iodobenzylguanidine in human neuroblastoma SK-N-SH cells. Cancer Res 49: 2941–2944.
Smith AS, Ågmo A, Birnie AK, French JA (2010). Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Hormones Behav 57: 255–262.
Štefánik P, Olexová L, Kršková L (2015). Increased sociability and gene expression of oxytocin and its receptor in the brains of rats affected prenatally by valproic acid. Pharmacol Biochem Behav 131: 42–50.
Turan T, Uysal C, Asdemir A, Kılıç E (2013). May oxytocin be a trait marker for bipolar disorder? Psychoneuroendocrinology 38: 2890–2896.
Uvnäsmoberg K, Alster P, Svensson TH (1992). Amperozide and clozapine but not haloperidol or raclopride increase the secretion of oxytocin in rats. Psychopharmacology 109: 473–476.
van de Nes JA, Kamphorst W, Ravid R, Swaab DF (1998). Comparison of beta-protein/A4 deposits and Alz-50-stained cytoskeletal changes in the hypothalamus and adjoining areas of Alzheimer's disease patients: amorphic plaques and cytoskeletal changes occur independently. Acta Neuropathol 96: 129–138.
Wade TD, Bulik CM, Neale M, Kendler KS (2000). Anorexia nervosa and major depression: shared genetic and environmental risk factors. Am J Psychiatry 157: 469–471.
Wang S, Kamphuis W, Huitinga I, Zhou J, Swaab D (2008). Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry 13: 786–799.
Watson GS (1982). Circular statistics in biology. Technometrics 24: 336–336.
Watts AG (2005). Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: a complexity beyond negative feedback. Front Neuroendocrinol 26: 109–130.
Wilson CM, McPhaul MJ (1996). A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120: 51–57.
Yagi K, Onaka T (1996). A benzodiazepine, chlordiazepoxide, blocks vasopressin and oxytocin release after footshocks but not osmotic stimulus in the rat. Neurosci Lett 203: 49–52.
Yasui T, Matsui S, Tani A, Kunimi K, Yamamoto S, Irahara M (2012). Androgen in postmenopausal women. J Med Investig 59: 12–27.
You ZD, Li JH, Song CY, Lu CL, He C (2001). Oxytocin mediates the inhibitory action of acute lithium on the morphine dependence in rats. Neurosci Res 41: 143–150.
Zhou L, Blaustein JD, De Vries GJ (1994). Distribution of androgen receptor immunoreactivity in vasopressin- and oxytocin-immunoreactive neurons in the male rat brain. Endocrinology 134: 2622–2627.
Acknowledgements
We are grateful to the Netherlands Brain Bank (Director Dr Inge Huitinga) for providing human brain material and clinical details, to Professor Joost Verhaagen for critically reading the manuscript, to Dr F.W. van Leeuwen for providing the OXT antibody, to Dr J. Trapman for providing the plasmid PSV-AR and to Ms W.T.P. Verweij for her secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Dai, D., Li, QC., Zhu, QB. et al. Direct Involvement of Androgen Receptor in Oxytocin Gene Expression: Possible Relevance for Mood Disorders. Neuropsychopharmacol 42, 2064–2071 (2017). https://doi.org/10.1038/npp.2017.76
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.76
This article is cited by
-
The impact of testosterone on paraventricular nucleus gene expression in male and female spontaneously hypertensive rats
Biology of Sex Differences (2026)
-
Elevated oxytocin levels and oxytocin receptor gene expression in female-to-male transgender individuals
Scientific Reports (2025)
-
Hypothalamic Oxytocin Neuronal Activation Induces Bipolar-Like Mood Changes in Mice in a Sex- and Dosage-Dependent Manner
Neuroscience Bulletin (2025)
-
Intranasal oxytocin interacts with testosterone reactivity to modulate parochial altruism
Communications Psychology (2024)
-
Therapeutic uses of oxytocin in stress-related neuropsychiatric disorders
Cell & Bioscience (2023)