Abstract
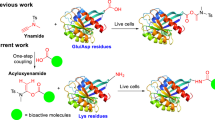
This protocol describes the preparation of Ab constructs using agents that target cells expressing integrins αvβ3 and αvβ5, and the monoclonal aldolase Ab 38C2. The targeting agents are equipped with a diketone or vinylketone linker, and selectively react through the reactive Lys residues in the Ab binding sites to form 38C2 conjugates or chemically programmed 38C2 (i.e., cp38C2). The targeting agent possessing a diketone linker reacts with the Lys residues forming an enaminone derivative. By contrast, the vinylketone linker is used as the corresponding acetone adduct (i.e., a pro-vinylketone linker), and this pro-adapter undergoes a 38C2-catalyzed retro-aldol reaction to produce the vinylketone linker, which forms a Michael-type adduct with the Lys residues. The Ab construct formation is achieved in <1 h for the diketone compounds at ambient temperature, and in 2–16 h using the pro-vinylketone linker at 37 °C. The 38C2 constructs are retargeted to cells over-expressing integrins, and are potential candidates for immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hale, G. Therapeutic antibodies: Delivering the promise? Adv. Drug Delivery Rev. 58, 633–639 (2006).
Kohzoh, I. & Akinori, T. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 6, 714–727 (2006).
Rader, C., Sinha, S.C., Popkov, M., Lerner, R.A. & Barbas, C.F., III A chemically programmed monoclonal antibody for cancer therapy. Adapter immunotherapy based on a covalent antibody catalyst. Proc. Natl. Acad. Sci. USA 100, 5396–5400 (2003).
Guo, F. et al. Breaking the one antibody one target axiom. Proc. Natl. Acad. Sci. USA 103, 11009–11014 (2006).
Popkov, M., Rader, C., Gonzalez, B., Sinha, S.C. & Barbas, C.F., III Small molecule drug activity in melanoma models may be dramatically enhanced with an antibody effector. Int. J. Cancer 119, 1194–1207 (2006).
Doppalaudi, V.R. et. al. Chemically programmed antibodies: Endothelin receptor targeting Cov X-Bodies. Biorg. Med. Chem. Lett. 17, 501–506 (2006).
Li, L.-S. et. al. Chemical-adaptor immunotherapy: design, synthesis and evaluation of novel integrin-targeting devices. J. Med. Chem. 47, 5630–5640 (2004).
Wagner, J., Lerner, R.A. & Barbas, C.F., III Efficient aldolase catalytic antibodies that use the enamine mechanism of natural enzymes. Science 270, 1797–1800 (1995).
Rader, C. et al. A humanized aldolase antibody for selective chemotherapy and adaptor immunotherapy. J. Mol. Biol. 332, 889–899 (2003).
Kok, R.J. et al. Preparation and functional evaluation of RGD-modified proteins as αvβ3 integrin directed therapeutics. Bioconjugate Chem. 13, 128–135 (2002).
Lu, Y. et al. Preclinical pharmacokinetics, tissue distribution, and antitumor activity of a folate-hapten conjugate-targeted immunotherapy in hapten-immunized mice. Mol. Cancer Ther. 5, 3258–3267 (2006).
Duggan, M.E. et al. Nonpeptide αvβ3 antagonists. 1. Transformation of a potent, integrin-selective αIIbβ3 antagonist into a potent αvβ3 antagonist. J. Med. Chem. 43, 3736–3745 (2000).
List, B., Barbas, C.F., III & Lerner, R.A. Aldol sensors for the rapid generation of tunable fluorescence by antibody catalysis. Proc. Natl. Acad. Sci. USA 95, 15351–15355 (1998).
Acknowledgements
The authors thank the Skaggs Institute for Chemical Biology and National Institutes of Health (grant number: RO1 CA120289 to S.C.S. and RO1CA104045 to C.F.B.) for financial support, C. Rader of the National Cancer Institute for helpful discussions and D. Kubitz for supplying Ab 38C2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Patents related to this work have been licensed to CovX, Inc. in which C.F.B., R.A.L., and S.C.S. maintain an equity position.
Rights and permissions
About this article
Cite this article
Sinha, S., Das, S., Li, LS. et al. Preparation of integrin α(v)β(3)-targeting Ab 38C2 constructs. Nat Protoc 2, 449–456 (2007). https://doi.org/10.1038/nprot.2007.3
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2007.3
This article is cited by
-
Harnessing a catalytic lysine residue for the one-step preparation of homogeneous antibody-drug conjugates
Nature Communications (2017)
-
Synthesis, Crystallographic and Spectral Studies of 3-(4-(Phenylamino) Phenylamino)Cyclohex-2-Enone
Journal of Chemical Crystallography (2010)